Stimulation of beta(3)-adrenoceptors causes phosphorylation of p38 mitogen-activated protein kinase via a stimulatory G protein-dependent pathway in 3T3-L1 adipocytes
- PMID: 11861323
- PMCID: PMC1573201
- DOI: 10.1038/sj.bjp.0704537
Stimulation of beta(3)-adrenoceptors causes phosphorylation of p38 mitogen-activated protein kinase via a stimulatory G protein-dependent pathway in 3T3-L1 adipocytes
Abstract
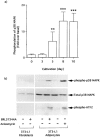
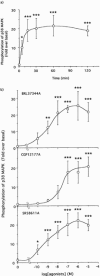
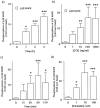
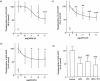
1. This study deals with phosphorylation and activation of p38 mitogen-activated protein kinase (MAPK) via beta(3)-adrenoceptor (AR) and the signal transduction pathway in 3T3-L1 adipocytes. 2. beta(3)-AR agonist BRL37344A (10 nM) caused phosphorylation and activation of p38 MAPK in 3T3-L1 adipocytes but not in fibroblasts. BRL37344A and also the other beta(3)-AR agonists, CGP12177A and SR58611A, caused p38 MAPK phosphorylation in dose-dependent manners. 3. The p38 MAPK phosphorylations by BRL37344A (10 nM), CGP12177A (100 nM), and SR58611A (10 nM) were not antagonized by beta(1)- and beta(2)-ARs antagonist 1-propranolol (100 nM) but blocked by beta(3)-AR antagonist SR59230A (10 microM), suggesting the phosphorylation was caused via beta(3)-AR. 4. The phosphorylations of p38 MAPK were completely abolished by treatment with cholera toxin (CTX) but not pertussis toxin (100 ng ml(-1), 24 h). Activation of Gs by CTX (100 ng ml(-1)) and adenylyl cyclase by forskolin mimicked p38 MAPK phosphorylation. 5. p38 MAPK phosphorylation by BRL37344A was reduced to almost 50% by cyclic AMP-dependent protein kinase (PKA) inhibitors such as H89 (10 microM) and PKI (10 microM). A src-family tyrosine kinases inhibitor PP2 (1 microM) also halved the p38 MAPK phosphorylation. Combined use of H89 (10 microM) and PP2 (10 microM) did not bring about further inhibition. 6. These results suggest that beta(3)-AR caused phosphorylation of p38 MAPK via Gs protein and partly through a pathway involving PKA and src-family kinase(s), although the contribution of the unidentified pathway remains to be clarified.
Figures


Similar articles
-
beta-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase.J Biol Chem. 2001 Jul 20;276(29):27077-82. doi: 10.1074/jbc.M101049200. Epub 2001 May 21. J Biol Chem. 2001. PMID: 11369767
-
Mouse beta 3a- and beta 3b-adrenoceptors expressed in Chinese hamster ovary cells display identical pharmacology but utilize distinct signalling pathways.Br J Pharmacol. 2002 Apr;135(8):1903-14. doi: 10.1038/sj.bjp.0704654. Br J Pharmacol. 2002. PMID: 11959793 Free PMC article.
-
The stimulation of beta(3)-adrenoceptor causes phosphorylation of extracellular signal-regulated kinases 1 and 2 through a G(s)- but not G(i)-dependent pathway in 3T3-L1 adipocytes.Eur J Pharmacol. 2000 Sep 15;404(1-2):63-8. doi: 10.1016/s0014-2999(00)00601-4. Eur J Pharmacol. 2000. PMID: 10980263
-
beta 2-adrenergic receptor-induced p38 MAPK activation is mediated by protein kinase A rather than by Gi or gbeta gamma in adult mouse cardiomyocytes.J Biol Chem. 2000 Dec 22;275(51):40635-40. doi: 10.1074/jbc.M006325200. J Biol Chem. 2000. PMID: 11018034
-
Modification of beta-adrenoceptor signal transduction pathway by genetic manipulation and heart failure.Mol Cell Biochem. 2000 Nov;214(1-2):131-55. doi: 10.1023/a:1007131925048. Mol Cell Biochem. 2000. PMID: 11195784 Review.
Cited by
-
Molecular pathways of oestrogen receptors and β-adrenergic receptors in cardiac cells: Recognition of their similarities, interactions and therapeutic value.Acta Physiol (Oxf). 2018 Feb;222(2):e12978. doi: 10.1111/apha.12978. Epub 2017 Oct 30. Acta Physiol (Oxf). 2018. PMID: 28994249 Free PMC article. Review.
-
Adrenaline increases glucose transport via a Rap1-p38MAPK pathway in rat vascular smooth muscle cells.Br J Pharmacol. 2007 Jun;151(4):476-82. doi: 10.1038/sj.bjp.0707247. Epub 2007 Apr 23. Br J Pharmacol. 2007. PMID: 17450172 Free PMC article.
-
Role of adenylyl cyclase in reduced β-adrenoceptor-mediated vasorelaxation during maturation.Braz J Med Biol Res. 2016 Jul 4;49(7):e5285. doi: 10.1590/1414-431X20165285. Braz J Med Biol Res. 2016. PMID: 27383122 Free PMC article.
-
Desensitization of cAMP Accumulation via Human β3-Adrenoceptors Expressed in Human Embryonic Kidney Cells by Full, Partial, and Biased Agonists.Front Pharmacol. 2019 Jun 7;10:596. doi: 10.3389/fphar.2019.00596. eCollection 2019. Front Pharmacol. 2019. PMID: 31263412 Free PMC article.
-
Ligand-directed signalling at beta-adrenoceptors.Br J Pharmacol. 2010 Mar;159(5):1022-38. doi: 10.1111/j.1476-5381.2009.00602.x. Epub 2010 Feb 2. Br J Pharmacol. 2010. PMID: 20132209 Free PMC article. Review.
References
-
- ANTHONSEN M.W., RONNSTRAND L., WERNSTEDT C., DEGERMAN E., HOLM C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoporterenol and govern activation properties in vitro. J. Biol. Chem. 1998;273:215–221. - PubMed
-
- ARCH J.R.S., ANISWORTH A.T., CAWTHORNE M.A., PIERCY V., SENNITT M.V., THODY V.E., WILSON C., WILSON S. Atypical β-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature. 1984;309:163–165. - PubMed
-
- CAO W., MEDVEDEV A.V., DANIEL K.W., COLLINS S. β-Adrenergic activation of p38 MAP kinase in adipocytes. J. Biol. Chem. 2001;276:27077–27082. - PubMed
-
- CAO W., LUTTEREL L.M., MEDVEDEV A.V., PIERCE K.L., DANIEL K.W., DIXON T.M., LEFKOWITZ R.J., COLLINS S. Direct binding of activated c-src to the β3-adrenergic receptor is required for MAP kinase activation. J. Biol. Chem. 2000;275:38131–38134. - PubMed
-
- CARR C., LONEY C., UNSON C., KNOWLER J., MILLIGAN G. Chronic exposure of rat glioma C6 cells to cholera toxin induces loss of the α-subunit of the stimulatory guanine nucleotide-binding protein (Gs) Eur. J. Pharmacol. 1990;188:203–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

