Purification and characterization of oligomeric envelope glycoprotein from a primary R5 subtype B human immunodeficiency virus
- PMID: 11861851
- PMCID: PMC135955
- DOI: 10.1128/jvi.76.6.2835-2847.2002
Purification and characterization of oligomeric envelope glycoprotein from a primary R5 subtype B human immunodeficiency virus
Abstract
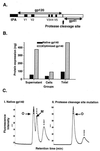
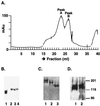
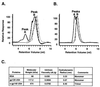
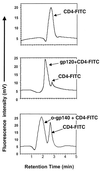
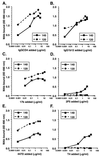
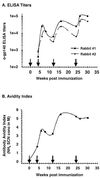
Human immunodeficiency virus (HIV) continues to be a major public health problem throughout the world, with high levels of mortality and morbidity associated with AIDS. Considerable efforts to develop an effective vaccine for HIV have been directed towards the generation of cellular, humoral, and mucosal immune responses. A major emphasis of our work has been toward the evaluation of oligomeric (o-gp140) forms of the HIV type 1 (HIV-1) envelope protein for their ability to induce neutralizing antibody responses. We have derived stable CHO cell lines expressing o-gp140 envelope protein from the primary non-syncytium-inducing (R5) subtype B strain HIV-1(US4). We have developed an efficient purification strategy to purify oligomers to near homogeneity. Using a combination of three detectors measuring intrinsic viscosity, light scattering, and refractive index, we calculated the molecular mass of the oligomer to be 474 kDa, consistent with either a trimer or a tetramer. The hydrodynamic radius (R(h)) of o-gp140 was determined to be 8.40 nm, compared with 5.07 nm for the monomer. The relatively smaller R(h) of the oligomer suggests that there are indeed differences between the foldings of o-gp140 and gp120. To assess the structural integrity of the purified trimers, we performed a detailed characterization of the glycosylation profile of o-gp140, its ability to bind soluble CD4, and also its ability to bind to a panel of monoclonal antibodies with known epitope specificities for the CD4 binding site, the CD4 inducible site, the V3 loop, and gp41. Immunogenicity studies with rabbits indicated that the purified o-gp140 protein was highly immunogenic and induced high-titer, high-avidity antibodies directed predominantly against conformational epitopes. These observations confirm the structural integrity of purified o-gp140 and its potential as a vaccine antigen.
Figures
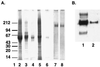


Similar articles
-
Purification, characterization, and immunogenicity of a soluble trimeric envelope protein containing a partial deletion of the V2 loop derived from SF162, an R5-tropic human immunodeficiency virus type 1 isolate.J Virol. 2003 Oct;77(20):11244-59. doi: 10.1128/jvi.77.20.11244-11259.2003. J Virol. 2003. PMID: 14512572 Free PMC article.
-
A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120.Virology. 2007 Apr 10;360(2):329-40. doi: 10.1016/j.virol.2006.10.032. Epub 2006 Nov 28. Virology. 2007. PMID: 17126869
-
Comparison of HIV Type 1 ADA gp120 monomers versus gp140 trimers as immunogens for the induction of neutralizing antibodies.AIDS Res Hum Retroviruses. 2005 Jan;21(1):58-67. doi: 10.1089/aid.2005.21.58. AIDS Res Hum Retroviruses. 2005. PMID: 15665645
-
Immunogenicity and protective efficacy of oligomeric human immunodeficiency virus type 1 gp140.J Virol. 2001 Jan;75(2):645-53. doi: 10.1128/JVI.75.2.645-653.2001. J Virol. 2001. PMID: 11134278 Free PMC article.
-
Rationale for a vaccine using cellular-derived epitope presented by HIV isolates.Vaccine. 1999 Mar 26;17(13-14):1700-5. doi: 10.1016/s0264-410x(98)00432-0. Vaccine. 1999. PMID: 10194825 Review.
Cited by
-
Immunogens Modeling a Fusion-Intermediate Conformation of gp41 Elicit Antibodies to the Membrane Proximal External Region of the HIV Envelope Glycoprotein.PLoS One. 2015 Jun 18;10(6):e0128562. doi: 10.1371/journal.pone.0128562. eCollection 2015. PLoS One. 2015. PMID: 26087072 Free PMC article.
-
Characterization of the outer domain of the gp120 glycoprotein from human immunodeficiency virus type 1.J Virol. 2004 Dec;78(23):12975-86. doi: 10.1128/JVI.78.23.12975-12986.2004. J Virol. 2004. PMID: 15542649 Free PMC article.
-
Robotic selection for the rapid development of stable CHO cell lines for HIV vaccine production.PLoS One. 2018 Aug 2;13(8):e0197656. doi: 10.1371/journal.pone.0197656. eCollection 2018. PLoS One. 2018. PMID: 30071025 Free PMC article.
-
Neutralizing antibody responses to subtype B and C adjuvanted HIV envelope protein vaccination in rabbits.Virology. 2009 Apr 25;387(1):147-56. doi: 10.1016/j.virol.2009.02.005. Epub 2009 Feb 27. Virology. 2009. PMID: 19249806 Free PMC article.
-
Alterations in the immunogenic properties of soluble trimeric human immunodeficiency virus type 1 envelope proteins induced by deletion or heterologous substitutions of the V1 loop.J Virol. 2010 Oct;84(19):9932-46. doi: 10.1128/JVI.00868-10. Epub 2010 Jul 21. J Virol. 2010. PMID: 20660181 Free PMC article.
References
-
- Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526-5540. - PMC - PubMed
-
- Barnett, S. W., S. Rajasekar, H. Legg, B. Doe, D. H. Fuller, J. R. Haynes, C. M. Walker, and K. S. Steimer. 1997. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine 15:869-873. - PubMed
-
- Barr, P. J., K. S. Steimer, E. A. Sabin, D. Parkes, C. George-Nascimento, J. C. Stephans, M. A. Powers, A. Gyenes, G. A. Van Nest, E. T. Miller, et al. 1987. Antigenicity and immunogenicity of domains of the human immunodeficiency virus (HIV) envelope polypeptide expressed in the yeast Saccharomyces cerevisiae. Vaccine 5:90-101. - PubMed
-
- Binley, J. M., H. Arshad, T. R. Fouts, and J. P. Moore. 1997. An investigation of the high-avidity antibody response to glycoprotein 120 of human immunodeficiency virus type 1 AIDS Res. Hum. Retroviruses 13:1007-1015. - PubMed
-
- Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

