Solution structure of the viral receptor domain of Tva and its implications in viral entry
- PMID: 11861852
- PMCID: PMC135981
- DOI: 10.1128/jvi.76.6.2848-2856.2002
Solution structure of the viral receptor domain of Tva and its implications in viral entry
Abstract
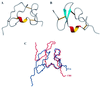
Tva is the cellular receptor for subgroup A avian sarcoma and leukosis virus (ASLV-A). The viral receptor function of Tva is determined by a 40-residue, cysteine-rich motif called the LDL-A module. Here we report the solution structure of the LDL-A module of Tva, determined by nuclear magnetic resonance (NMR) spectroscopy. Although the carboxyl terminus of the Tva LDL-A module has a structure similar to those of other reported LDL-A modules, the amino terminus adopts a different conformation. The LDL-A module of Tva does not contain the signature antiparallel beta-sheet observed in other LDL-A modules, and it is more flexible than other reported LDL-A modules. The LDL-A structure of Tva provides mechanistic insights into how a simple viral receptor functions in retrovirus entry. The side chains of H38 and W48 of Tva, which have been identified as viral contact residues by mutational analysis, are solvent exposed, suggesting that they are directly involved in EnvA binding. However, the side chain of L34, another potential viral contact residue identified previously, is buried inside of the module and forms the hydrophobic core with other residues. Thus L34 likely stabilizes the Tva structure but is not a viral interaction determinant. In addition, we propose that the flexible amino-terminal region of Tva plays an important role in determining specificity in the Tva-EnvA interaction.
Figures
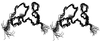
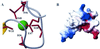


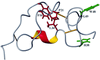
Similar articles
-
Identification of two residues within the LDL-A module of Tva that dictate the altered receptor specificity of mutant subgroup A avian sarcoma and leukosis viruses.J Virol. 2005 Dec;79(23):14962-6. doi: 10.1128/JVI.79.23.14962-14966.2005. J Virol. 2005. PMID: 16282495 Free PMC article.
-
The spacing between cysteines two and three of the LDL-A module of Tva is important for subgroup A avian sarcoma and leukosis virus entry.J Virol. 2004 Jan;78(2):683-91. doi: 10.1128/jvi.78.2.683-691.2004. J Virol. 2004. PMID: 14694099 Free PMC article.
-
Role of calcium in protein folding and function of Tva, the receptor of subgroup A avian sarcoma and leukosis virus.J Virol. 2001 Mar;75(5):2051-8. doi: 10.1128/JVI.75.5.2051-2058.2001. J Virol. 2001. PMID: 11160709 Free PMC article.
-
Characterization of determinants for envelope binding and infection in tva, the subgroup A avian sarcoma and leukosis virus receptor.J Virol. 1998 Jun;72(6):4552-9. doi: 10.1128/JVI.72.6.4552-4559.1998. J Virol. 1998. PMID: 9573218 Free PMC article.
-
Avian sarcoma and leukosis virus-receptor interactions: from classical genetics to novel insights into virus-cell membrane fusion.Virology. 2006 Jan 5;344(1):25-9. doi: 10.1016/j.virol.2005.09.021. Virology. 2006. PMID: 16364732 Review.
Cited by
-
A mutation in the first ligand-binding repeat of the human very-low-density lipoprotein receptor results in high-affinity binding of the single V1 module to human rhinovirus 2.J Virol. 2005 Dec;79(23):14730-6. doi: 10.1128/JVI.79.23.14730-14736.2005. J Virol. 2005. PMID: 16282473 Free PMC article.
-
Identification of two residues within the LDL-A module of Tva that dictate the altered receptor specificity of mutant subgroup A avian sarcoma and leukosis viruses.J Virol. 2005 Dec;79(23):14962-6. doi: 10.1128/JVI.79.23.14962-14966.2005. J Virol. 2005. PMID: 16282495 Free PMC article.
-
Two different molecular defects in the Tva receptor gene explain the resistance of two tvar lines of chickens to infection by subgroup A avian sarcoma and leukosis viruses.J Virol. 2004 Dec;78(24):13489-500. doi: 10.1128/JVI.78.24.13489-13500.2004. J Virol. 2004. PMID: 15564460 Free PMC article.
-
Characterization of the LDL-A module mutants of Tva, the subgroup A Rous sarcoma virus receptor, and the implications in protein folding.Protein Sci. 2002 Nov;11(11):2596-605. doi: 10.1110/ps.0219802. Protein Sci. 2002. PMID: 12381843 Free PMC article.
-
The mature avian leukosis virus subgroup A envelope glycoprotein is metastable, and refolding induced by the synergistic effects of receptor binding and low pH is coupled to infection.J Virol. 2004 Feb;78(3):1403-10. doi: 10.1128/jvi.78.3.1403-1410.2004. J Virol. 2004. PMID: 14722295 Free PMC article.
References
-
- Bates, P., J. A. T. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74:1043-1051. - PubMed
-
- Daly, N. L., J. T. Djordjevic, P. A. Kroon, and R. Smith. 1995. Three-dimensional structure of the second cysteine-rich repeat from the human low-density lipoprotein receptor. Biochemistry 34:14474-14481. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

