Distinct glycoprotein O complexes arise in a post-Golgi compartment of cytomegalovirus-infected cells
- PMID: 11861856
- PMCID: PMC135985
- DOI: 10.1128/jvi.76.6.2890-2898.2002
Distinct glycoprotein O complexes arise in a post-Golgi compartment of cytomegalovirus-infected cells
Abstract
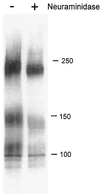
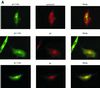
Human cytomegalovirus (CMV) glycoproteins H, L, and O (gH, gL, and gO, respectively) form a heterotrimeric disulfide-bonded complex that participates in the fusion of the viral envelope with the host cell membrane. During virus maturation, this complex undergoes a series of intracellular assembly and processing events which are not entirely defined (M. T. Huber and T. Compton, J. Virol. 73:3886-3892, 1999). Here, we demonstrate that gO does not undergo the same posttranslational processing in transfected cells as it does in infected cells. We further determined that gO is modified by O-linked glycosylation and that this terminally processed form is highly enriched in virions. However, during studies of gO processing, novel gO complexes were discovered in CMV virions. The newly identified gO complexes, including gO-gL heterodimers, were not readily detected in CMV-infected cells. Further characterization of the trafficking of gO through the secretory pathway of infected cells localized gH, gL, and gO primarily to the Golgi apparatus and trans-Golgi network, supporting the conclusion that the novel virion-associated gO complexes arise in a post-Golgi compartment of infected cells.
Figures
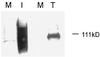


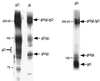



Similar articles
-
Human cytomegalovirus TR strain glycoprotein O acts as a chaperone promoting gH/gL incorporation into virions but is not present in virions.J Virol. 2010 Mar;84(5):2597-609. doi: 10.1128/JVI.02256-09. Epub 2009 Dec 23. J Virol. 2010. PMID: 20032193 Free PMC article.
-
The Human Cytomegalovirus Protein UL116 Interacts with the Viral Endoplasmic-Reticulum-Resident Glycoprotein UL148 and Promotes the Incorporation of gH/gL Complexes into Virions.J Virol. 2021 Jul 12;95(15):e0220720. doi: 10.1128/JVI.02207-20. Epub 2021 Jul 12. J Virol. 2021. PMID: 34011552 Free PMC article.
-
Intracellular formation and processing of the heterotrimeric gH-gL-gO (gCIII) glycoprotein envelope complex of human cytomegalovirus.J Virol. 1999 May;73(5):3886-92. doi: 10.1128/JVI.73.5.3886-3892.1999. J Virol. 1999. PMID: 10196283 Free PMC article.
-
Principles for studying in vivo attenuation of virus mutants: defining the role of the cytomegalovirus gH/gL/gO complex as a paradigm.Med Microbiol Immunol. 2015 Jun;204(3):295-305. doi: 10.1007/s00430-015-0405-2. Epub 2015 Mar 18. Med Microbiol Immunol. 2015. PMID: 25782576 Review.
-
Characteristics and functions of human cytomegalovirus UL128 gene/protein.Acta Virol. 2014;58(2):103-7. doi: 10.4149/av_2014_02_103. Acta Virol. 2014. PMID: 24957713 Review.
Cited by
-
The endoplasmic reticulum chaperone BiP/GRP78 is important in the structure and function of the human cytomegalovirus assembly compartment.J Virol. 2009 Nov;83(22):11421-8. doi: 10.1128/JVI.00762-09. Epub 2009 Sep 9. J Virol. 2009. PMID: 19741001 Free PMC article.
-
Human Cytomegalovirus pUL47 Modulates Tegumentation and Capsid Accumulation at the Viral Assembly Complex.J Virol. 2015 Jul;89(14):7314-28. doi: 10.1128/JVI.00603-15. Epub 2015 May 6. J Virol. 2015. PMID: 25948747 Free PMC article.
-
Rhesus and human cytomegalovirus glycoprotein L are required for infection and cell-to-cell spread of virus but cannot complement each other.J Virol. 2011 Mar;85(5):2089-99. doi: 10.1128/JVI.01970-10. Epub 2010 Dec 29. J Virol. 2011. PMID: 21191007 Free PMC article.
-
Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2.J Virol. 2003 Apr;77(8):4588-96. doi: 10.1128/jvi.77.8.4588-4596.2003. J Virol. 2003. PMID: 12663765 Free PMC article.
-
Human cytomegalovirus infection maintains mTOR activity and its perinuclear localization during amino acid deprivation.J Virol. 2011 Sep;85(18):9369-76. doi: 10.1128/JVI.05102-11. Epub 2011 Jul 6. J Virol. 2011. PMID: 21734039 Free PMC article.
References
-
- Ahmed, H., and B. P. Chatterjee. 1989. Further characterization and immunochemical studies on the carbohydrate specificity of jackfruit (Artocarpus integrifolia) lectin. J. Biol. Chem. 264:9365-9372. - PubMed
-
- Alford, C. A., and W. J. Britt. 1993. Cytomegalovirus, p. 227-255. In B. Roizman, R. J. Whitley, and C. Lopez (ed.), Human herpesviruses. Raven Press, New York, N.Y.
-
- Bendjennat, M., B. Bahbouhi, and E. Bahraoui. 2001. Purification and characterization of a Ca2+-independent endoprotease activity from peripheral blood lymphocytes: involvement in HIV-1 gp160 maturation. Biochemistry 40:4800-4810. - PubMed
-
- Bogner, E., M. Reschke, B. Reis, E. Reis, W. Britt, and K. Radsak. 1992. Recognition of compartmentalized intracellular analogs of glycoprotein H of human cytomegalovirus. Arch. Virol. 126:67-80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

