Distinct roles of adenovirus vector-transduced dendritic cells, myoblasts, and endothelial cells in mediating an immune response against a transgene product
- PMID: 11861857
- PMCID: PMC136003
- DOI: 10.1128/jvi.76.6.2899-2911.2002
Distinct roles of adenovirus vector-transduced dendritic cells, myoblasts, and endothelial cells in mediating an immune response against a transgene product
Abstract
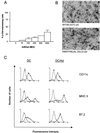
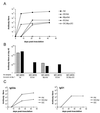
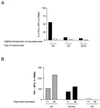
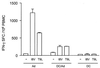
Adenovirus-mediated gene delivery via the intramuscular route efficiently promotes an immune response against the transgene product. In this study, a recombinant adenovirus vector encoding beta-galactosidase (Ad beta Gal) was used to transduce dendritic cells (DC), which are antigen-presenting cells, as well as myoblasts and endothelial cells (EC), neither of which present antigens. C57BL/6 mice received a single intramuscular injection of Ad beta Gal-transduced DC, EC, or myoblasts and were then monitored for anti-beta-galactosidase (anti-beta-Gal) antibody production, induction of gamma interferon-secreting CD8(+) T cells, and protection against melanoma tumor cells expressing beta-Gal. While all transduced cell types were able to elicit an antibody response against the transgene product, the specific isotypes were distinct, with exclusive production of immunoglobulin G2a (IgG2a) antibodies following injection of transduced DC and EC versus equivalent IgG1 and IgG2a responses in mice inoculated with transduced myoblasts. Transduced DC induced a strong ex vivo CD8(+) T-cell response at a level of 50% of the specific response obtained with the Ad beta Gal control. In contrast, this response was 6- to 10-fold-lower in animals injected with transduced myoblasts and EC. Accordingly, only animals injected with transduced DC were protected against a beta-Gal tumor challenge. Thus, in order to induce a strong and protective immune response to an adenovirus-encoded transgene product, it is necessary to transduce cells of dendritic lineage. Importantly, it will be advantageous to block the transduction of DC for adenovirus-based gene therapy strategies.
Figures




Similar articles
-
Modification to the capsid of the adenovirus vector that enhances dendritic cell infection and transgene-specific cellular immune responses.J Virol. 2004 Mar;78(5):2572-80. doi: 10.1128/jvi.78.5.2572-2580.2004. J Virol. 2004. PMID: 14963160 Free PMC article.
-
Adenoviral transgene ubiquitination enhances mouse immunization and class I presentation by human dendritic cells.Hum Gene Ther. 2003 Sep 20;14(14):1319-32. doi: 10.1089/104303403322319408. Hum Gene Ther. 2003. PMID: 14503967
-
Dendritic cell-based genetic immunization in mice with a recombinant adenovirus encoding murine TRP2 induces effective anti-melanoma immunity.J Gene Med. 1999 Nov-Dec;1(6):400-6. doi: 10.1002/(SICI)1521-2254(199911/12)1:6<400::AID-JGM68>3.0.CO;2-D. J Gene Med. 1999. PMID: 10753065
-
The role of chromatin in adenoviral vector function.Viruses. 2013 Jun 14;5(6):1500-15. doi: 10.3390/v5061500. Viruses. 2013. PMID: 23771241 Free PMC article. Review.
-
Innate immune response to adenovirus.J Investig Med. 2005 Sep;53(6):292-304. doi: 10.2310/6650.2005.53605. J Investig Med. 2005. PMID: 16207466 Review.
Cited by
-
Gene Therapy Applications to Cancer Treatment.J Biomed Biotechnol. 2003;2003(1):35-47. doi: 10.1155/S1110724303209037. J Biomed Biotechnol. 2003. PMID: 12686721 Free PMC article.
-
Limited expression of non-integrating CpG-free plasmid is associated with increased nucleosome enrichment.PLoS One. 2020 Dec 21;15(12):e0244386. doi: 10.1371/journal.pone.0244386. eCollection 2020. PLoS One. 2020. PMID: 33347482 Free PMC article.
-
The gut microbiota modifies antibody durability and booster responses after SARS-CoV-2 vaccination.J Transl Med. 2024 Sep 6;22(1):827. doi: 10.1186/s12967-024-05637-2. J Transl Med. 2024. PMID: 39242525 Free PMC article.
-
Breaking Entry-and Species Barriers: LentiBOOST® Plus Polybrene Enhances Transduction Efficacy of Dendritic Cells and Monocytes by Adenovirus 5.Viruses. 2022 Jan 5;14(1):92. doi: 10.3390/v14010092. Viruses. 2022. PMID: 35062296 Free PMC article.
-
In vivo tracking of adenoviral-transduced iron oxide-labeled bone marrow-derived dendritic cells using magnetic particle imaging.Eur Radiol Exp. 2023 Aug 15;7(1):42. doi: 10.1186/s41747-023-00359-4. Eur Radiol Exp. 2023. PMID: 37580614 Free PMC article.
References
-
- Acsadi, G., A. Jani, J. Huard, K. Blaschuk, B. Massie, P. Holland, H. Lochmuller, and G. Karpati. 1994. Cultured human myoblasts and myotubes show markedly different transducibility by replication-defective adenovirus recombinants. Gene Ther. 1:338-340. - PubMed
-
- Agadjanyan, M. G., J. J. Kim, N. Trivedi, D. M. Wilson, B. Monzavi-Karbassi, L. D. Morrison, L. K. Nottingham, T. Dentchev, A. Tsai, K. Dang, A. A. Chalian, M. A. Maldonado, V. W. Williams, and D. B. Weiner. 1999. CD86 (B7-2) can function to drive MHC-restricted antigen-specific CTL responses in vivo. J. Immunol. 162:3417-3427. - PubMed
-
- Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. - PubMed
-
- Ali, M., N. R. Lemoine, and C. J. Ring. 1994. The use of DNA viruses as vectors for gene therapy. Gene Ther. 1:367-384. - PubMed
-
- Ambriovic, A., M. Adam, M. Monteil, D. Paulin, and M. Eloit. 1997. Efficacy of replication-defective adenovirus-vectored vaccines: protection following intramuscular injection is linked to promoter efficiency in muscle representative cells. Virology 238:327-335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

