Single ion occupancy and steady-state gating of Na channels in squid giant axon
- PMID: 11865020
- PMCID: PMC2217285
- DOI: 10.1085/jgp.20028500
Single ion occupancy and steady-state gating of Na channels in squid giant axon
Erratum in
- J Gen Physiol 2002 Apr;119(4):391
Abstract
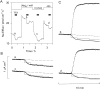
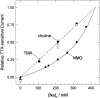
The properties of the small fraction of tetrodotoxin (TTX)-sensitive Na channels that remain open in the steady state were studied in internally dialyzed voltage clamped squid giant axons. The observed Ussing flux ratio exponent (n') of 0.97 plus minus 0.03 (calculated from simultaneous measurements of TTX-sensitive current and (22)Na efflux) and nonindependent behavior of Na current at high internal [Na] are explained by a one-site ("1s") permeation model characterized by a single effective binding site within the channel pore in equilibrium with internal Na ions (apparent equilibrium dissociation constant K(Nai)(0) = 0.61 +/- 0.08 M). Steady-state open probability of the TTX-sensitive channels can be modeled by the product p(a)p(infinity), where p(a) represents voltage-dependent activation described by a Boltzmann distribution with midpoint V(a) = -7 mV and effective valence z(a) = 3.2 (Vandenberg, C.A., and F. Bezanilla. 1991. BIOPHYS: J. 60:1499--1510) coupled to voltage-independent inactivation by an equilibrium constant (Bezanilla, F., and C.M. Armstrong. 1977. J. Gen. Physiol. 70:549--566) K(eq) = 770. The factor p(infinity) represents voltage-dependent inactivation with empirical midpoint V(infinity)= -83 plus minus 5 mV and effective valence z(infinity) = 0.55 plus minus 0.03. The composite p(a)p(infinity)1s model describes the steady-state voltage dependence of the persistent TTX-sensitive current well.
Figures








Similar articles
-
Stoichiometry and voltage dependence of the sodium pump in voltage-clamped, internally dialyzed squid giant axon.J Gen Physiol. 1989 May;93(5):903-41. doi: 10.1085/jgp.93.5.903. J Gen Physiol. 1989. PMID: 2544655 Free PMC article.
-
On the persistent sodium current in squid giant axons.J Neurophysiol. 2003 Jan;89(1):640-4. doi: 10.1152/jn.00652.2002. J Neurophysiol. 2003. PMID: 12522209
-
Properties of the voltage sensor for the opening and closing of the sodium channels in the squid giant axon.Proc Biol Sci. 1993 Jul 22;253(1336):61-8. doi: 10.1098/rspb.1993.0082. Proc Biol Sci. 1993. PMID: 8396778
-
Studies of multimodal gating of the sodium channel.Novartis Found Symp. 2002;241:5-14; discussion 14-20, 226-32. Novartis Found Symp. 2002. PMID: 11771650 Review.
-
Potassium current in the squid giant axon.Int Rev Neurobiol. 1985;27:363-84. doi: 10.1016/s0074-7742(08)60562-0. Int Rev Neurobiol. 1985. PMID: 2417975 Review.
Cited by
-
Steric selectivity in Na channels arising from protein polarization and mobile side chains.Biophys J. 2007 Sep 15;93(6):1960-80. doi: 10.1529/biophysj.107.105478. Epub 2007 May 25. Biophys J. 2007. PMID: 17526571 Free PMC article.
-
Sodium flux ratio in Na/K pump-channels opened by palytoxin.J Gen Physiol. 2007 Jul;130(1):41-54. doi: 10.1085/jgp.200709770. Epub 2007 Jun 11. J Gen Physiol. 2007. PMID: 17562821 Free PMC article.
-
Persistent tetrodotoxin-sensitive sodium current resulting from U-to-C RNA editing of an insect sodium channel.Proc Natl Acad Sci U S A. 2004 Aug 10;101(32):11862-7. doi: 10.1073/pnas.0307695101. Epub 2004 Jul 27. Proc Natl Acad Sci U S A. 2004. PMID: 15280550 Free PMC article.
-
Current and selectivity in a model sodium channel under physiological conditions: Dynamic Monte Carlo simulations.Biochim Biophys Acta. 2012 Mar;1818(3):592-600. doi: 10.1016/j.bbamem.2011.10.029. Epub 2011 Nov 4. Biochim Biophys Acta. 2012. PMID: 22080102 Free PMC article.
-
Sodium channel biophysics, late sodium current and genetic arrhythmic syndromes.Pflugers Arch. 2017 Jun;469(5-6):629-641. doi: 10.1007/s00424-017-1959-1. Epub 2017 Mar 6. Pflugers Arch. 2017. PMID: 28265756 Free PMC article. Review.
References
-
- Begenisich, T. 1987. Molecular properties of ion permeation through sodium channel. Annu. Rev. Biophys. Biophys. Chem. 16:247–263. - PubMed

