The endothelial receptor tyrosine kinase Tie1 activates phosphatidylinositol 3-kinase and Akt to inhibit apoptosis
- PMID: 11865050
- PMCID: PMC135589
- DOI: 10.1128/MCB.22.6.1704-1713.2002
The endothelial receptor tyrosine kinase Tie1 activates phosphatidylinositol 3-kinase and Akt to inhibit apoptosis
Abstract
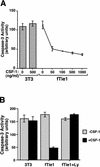
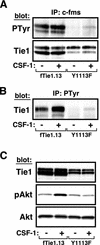
Tie1 is an orphan receptor tyrosine kinase that is expressed almost exclusively in endothelial cells and that is required for normal embryonic vascular development. Genetic studies suggest that Tie1 promotes endothelial cell survival, but other studies have suggested that the Tie1 kinase has little to no activity, and Tie1-mediated signaling pathways are unknown. To begin to study Tie1 signaling, a recombinant glutathione S-transferase (GST)-Tie1 kinase fusion protein was produced in insect cells and found to be autophosphorylated in vitro. GST-Tie1 but not a kinase-inactive mutant associated with a recombinant p85 SH2 domain protein in vitro, suggesting that Tie1 might signal through phosphatidylinositol (PI) 3-kinase. To study Tie1 signaling in a cellular context, a c-fms-Tie1 chimeric receptor (fTie1) was expressed in NIH 3T3 cells. Ligand stimulation of fTie1 resulted in Tie1 autophosphorylation and downstream activation of PI 3-kinase and Akt. Stimulation of fTie1-expressing cells potently inhibited UV irradiation-induced apoptosis in a PI 3-kinase-dependent manner. Moreover, both Akt phosphorylation and inhibition of apoptosis were abrogated by mutation of tyrosine 1113 to phenylalanine, suggesting that this residue is an important PI 3-kinase binding site. These findings are the first biochemical demonstration of a signal transduction pathway and corresponding cellular function for Tie1, and the antiapoptotic effect of Tie1 is consistent with the results of previous genetic studies.
Figures


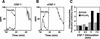



References
-
- Carpenter, C. L., and L. C. Cantley. 1996. Phosphoinositide kinases. Curr. Opin. Cell Biol. 8:153-158. - PubMed
-
- Davis, S., T. H. Aldrich, P. F. Jones, A. Acheson, D. L. Compton, V. Jain, T. E. Ryan, J. Bruno, C. Radziejewski, P. C. Maisonpierre, and G. D. Yancopoulos. 1996. Isolation of angiopoietin-1, a ligand for the Tie-2 receptor, by secretion-trap expression cloning. Cell 87:1161-1169. - PubMed
-
- Downward, J. 1998. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10:262-267. - PubMed
-
- Dubois, N. A., L. C. Kolpack, R. Wang, R. G. Azizkhan, and V. L. Bautch. 1991. Isolation and characterization of an established endothelial cell line from transgenic mouse hemangiomas. Exp. Cell Res. 196:302-313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
