IAP suppression of apoptosis involves distinct mechanisms: the TAK1/JNK1 signaling cascade and caspase inhibition
- PMID: 11865055
- PMCID: PMC135597
- DOI: 10.1128/MCB.22.6.1754-1766.2002
IAP suppression of apoptosis involves distinct mechanisms: the TAK1/JNK1 signaling cascade and caspase inhibition
Abstract
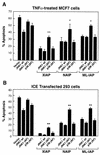
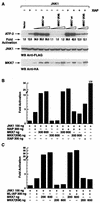
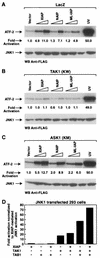
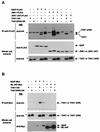
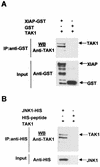
The antiapoptotic properties of the inhibitor of apoptosis (IAP) family of proteins have been linked to caspase inhibition. We have previously described an alternative mechanism of XIAP inhibition of apoptosis that depends on the selective activation of JNK1. Here we report that two other members of the IAP family, NAIP and ML-IAP, both activate JNK1. Expression of catalytically inactive JNK1 blocks NAIP and ML-IAP protection against ICE- and TNF-alpha-induced apoptosis, indicating that JNK1 activation is necessary for the antiapoptotic effect of these proteins. The MAP3 kinase, TAK1, appears to be an essential component of this antiapoptotic pathway since IAP-mediated activation of JNK1, as well as protection against TNF-alpha- and ICE-induced apoptosis, is inhibited when catalytically inactive TAK1 is expressed. In addition, XIAP, NAIP, and JNK1 bind to TAK1. Importantly, expression of catalytically inactive TAK1 did not affect XIAP inhibition of caspase activity. These data suggest that XIAP's antiapoptotic activity is achieved by two separate mechanisms: one requiring TAK1-dependent JNK1 activation and the second involving caspase inhibition.
Figures





References
-
- Ambrosini, G., C. Adida, and D. C. Altieri. 1997. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 3:917-921. - PubMed
-
- Bernardi, P., L. Scorrano, R. Colonna, V. Petronilli, and F. Di Lisa. 1999. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur. J. Biochem. 264:687-701. - PubMed
-
- Chen, Y. R., and T. H. Tan. 2000. The c-Jun N-terminal kinase pathway and apoptotic signaling. Int. J. Oncol. 16:651-662. - PubMed
-
- Decaudin, D., I. Marzo, C. Brenner, and G. Kroemer. 1998. Mitochondria in chemotherapy-induced apoptosis: a prospective novel target of cancer therapy. Int. J. Oncol. 12:141-152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
