A novel and conserved protein-protein interaction domain of mammalian Lin-2/CASK binds and recruits SAP97 to the lateral surface of epithelia
- PMID: 11865057
- PMCID: PMC135599
- DOI: 10.1128/MCB.22.6.1778-1791.2002
A novel and conserved protein-protein interaction domain of mammalian Lin-2/CASK binds and recruits SAP97 to the lateral surface of epithelia
Abstract
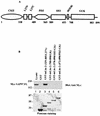
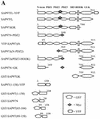
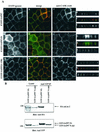
Mammalian Lin-2 (mLin-2)/CASK is a membrane-associated guanylate kinase (MAGUK) and contains multidomain modules that mediate protein-protein interactions important for the establishment and maintenance of neuronal and epithelial cell polarization. The importance of mLin-2/CASK in mammalian development is demonstrated by the fact that mutations in mLin-2/CASK or SAP97, another MAGUK protein, lead to cleft palate in mice. We recently identified a new protein-protein interaction domain, called the L27 domain, which is present twice in mLin-2/CASK. In this report, we further define the binding of the L27C domain of mLin-2/CASK to the L27 domain of mLin-7 and identify the binding partner for L27N of mLin-2/CASK. Biochemical analysis reveals that this L27N domain binds to the N terminus of SAP97, a region that was previously reported to be essential for the lateral membrane recruitment of SAP97 in epithelia. Our colocalization studies, using dominant-negative mLin-2/CASK, show that the association with mLin-2/CASK is crucial for lateral localization of SAP97 in MDCK cells. We also report the identification of a novel isoform of Discs Large, a Drosophila melanogaster orthologue of SAP97, which contains a region highly related to the SAP97 N terminus and which binds Camguk, a Drosophila orthologue of mLin-2/CASK. Our data identify evolutionarily conserved protein-protein interaction domains that link mLin-2/CASK to SAP97 and account for their common phenotype when mutated in mice.
Figures

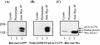







Similar articles
-
Identification of multiple binding partners for the amino-terminal domain of synapse-associated protein 97.J Biol Chem. 2002 Nov 29;277(48):46730-5. doi: 10.1074/jbc.M208781200. Epub 2002 Sep 25. J Biol Chem. 2002. PMID: 12351654
-
Molecular analysis of the X11-mLin-2/CASK complex in brain.J Neurosci. 1999 Feb 15;19(4):1307-16. doi: 10.1523/JNEUROSCI.19-04-01307.1999. J Neurosci. 1999. PMID: 9952408 Free PMC article.
-
CASK participates in alternative tripartite complexes in which Mint 1 competes for binding with caskin 1, a novel CASK-binding protein.J Neurosci. 2002 Jun 1;22(11):4264-73. doi: 10.1523/JNEUROSCI.22-11-04264.2002. J Neurosci. 2002. PMID: 12040031 Free PMC article.
-
The role of the MAGUK protein CASK in neural development and synaptic function.Curr Med Chem. 2006;13(16):1915-27. doi: 10.2174/092986706777585040. Curr Med Chem. 2006. PMID: 16842202 Review.
-
Genetic studies define MAGUK proteins as regulators of epithelial cell polarity.Int J Dev Biol. 2002;46(4):511-8. Int J Dev Biol. 2002. PMID: 12141438 Review.
Cited by
-
Presynaptic DLG regulates synaptic function through the localization of voltage-activated Ca(2+) Channels.Sci Rep. 2016 Aug 30;6:32132. doi: 10.1038/srep32132. Sci Rep. 2016. PMID: 27573697 Free PMC article.
-
Colocalization of APC and DLG at the tips of cellular protrusions in cultured epithelial cells and its dependency on cytoskeletons.Histochem Cell Biol. 2005 Jan;123(1):67-73. doi: 10.1007/s00418-004-0729-2. Epub 2004 Dec 18. Histochem Cell Biol. 2005. PMID: 15609045
-
Loss of Dlg-1 in the mouse lens impairs fibroblast growth factor receptor signaling.PLoS One. 2014 May 13;9(5):e97470. doi: 10.1371/journal.pone.0097470. eCollection 2014. PLoS One. 2014. PMID: 24824078 Free PMC article.
-
GluR1 controls dendrite growth through its binding partner, SAP97.J Neurosci. 2008 Oct 8;28(41):10220-33. doi: 10.1523/JNEUROSCI.3434-08.2008. J Neurosci. 2008. PMID: 18842882 Free PMC article.
-
CASK regulates SAP97 conformation and its interactions with AMPA and NMDA receptors.J Neurosci. 2013 Jul 17;33(29):12067-76. doi: 10.1523/JNEUROSCI.0816-13.2013. J Neurosci. 2013. PMID: 23864692 Free PMC article.
References
-
- Adey, N. B., L. Huang, P. A. Ormonde, M. L. Baumgard, R. Pero, D. V. Byreddy, S. V. Tavtigian, and P. L. Bartel. 2000. Threonine phosphorylation of the MMAC1/PTEN PDZ binding domain both inhibits and stimulates PDZ binding. Cancer Res. 60:35-37. - PubMed
-
- Borg, J. P., S. W. Straight, S. M. Kaech, M. de Taddeo-Borg, D. E. Kroon, D. Karnak, R. S. Turner, S. K. Kim, and B. Margolis. 1998. Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting. J. Biol. Chem. 273:31633-31636. - PubMed
-
- Budnik, V. 1996. Synapse maturation and structural plasticity at Drosophila neuromuscular junctions. Curr. Opin. Neurobiol. 6:858-867. - PubMed
-
- Butz, S., M. Okamoto, and T. C. Sudhof. 1998. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell 94:773-782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
