Diverse effects of mutations in exon II of the von Hippel-Lindau (VHL) tumor suppressor gene on the interaction of pVHL with the cytosolic chaperonin and pVHL-dependent ubiquitin ligase activity
- PMID: 11865071
- PMCID: PMC135590
- DOI: 10.1128/MCB.22.6.1947-1960.2002
Diverse effects of mutations in exon II of the von Hippel-Lindau (VHL) tumor suppressor gene on the interaction of pVHL with the cytosolic chaperonin and pVHL-dependent ubiquitin ligase activity
Abstract
We examined the biogenesis of the von Hippel-Lindau (VHL) tumor suppressor protein (pVHL) in vitro and in vivo. pVHL formed a complex with the cytosolic chaperonin containing TCP-1 (CCT or TRiC) en route to assembly with elongin B/C and the subsequent formation of the VCB-Cul2 ubiquitin ligase. Blocking the interaction of pVHL with elongin B/C resulted in accumulation of pVHL within the CCT complex. pVHL present in purified VHL-CCT complexes, when added to rabbit reticulocyte lysate, proceeded to form VCB and VCB-Cul2. Thus, CCT likely functions, at least in part, by retaining VHL chains pending the availability of elongin B/C for final folding and/or assembly. Tumor-associated mutations within exon II of the VHL syndrome had diverse effects upon the stability and/or function of pVHL-containing complexes. First, a pVHL mutant lacking the entire region encoded by exon II did not bind to CCT and yet could still assemble into complexes with elongin B/C and elongin B/C-Cul2. Second, a number of tumor-derived missense mutations in exon II did not decrease CCT binding, and most had no detectable effect upon VCB-Cul2 assembly. Many exon II mutants, however, were found to be defective in the binding to and subsequent ubiquitination of hypoxia-inducible factor 1alpha (HIF-1alpha), a substrate of the VCB-Cul2 ubiquitin ligase. We conclude that the selection pressure to mutate VHL exon II during tumorigenesis does not relate to loss of CCT binding but may reflect quantitative or qualitative defects in HIF binding and/or in pVHL-dependent ubiquitin ligase activity.
Figures





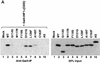
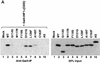
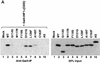

References
-
- Blankenship, C., J. G. Naglich, J. M. Whaley, B. Seizinger, and N. Kley. 1999. Alternate choice of initiation codon produces a biologically active product of the von Hippel Lindau gene with tumor suppressor activity. Oncogene 18:1529-1535. - PubMed
-
- Bonicalzi, M. E., I. Groulx, N. de Paulsen, and S. Lee. 2001. Role of exon 2-encoded β-domain of the von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 276:1407-1416. - PubMed
-
- Cockman, M. E., N. Masson, D. R. Mole, P. Jaakkola, G. W. Chang, S. C. Clifford, E. R. Maher, C. W. Pugh, P. J. Ratcliffe, and P. H. Maxwell. 2000. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 275:25733-25741. - PubMed
-
- Cowan, N. J., and S. A. Lewis. 1999. A chaperone with a hydrophilic surface. Nat. Struct. Biol. 6:990-991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
