Conversion of raft associated prion protein to the protease-resistant state requires insertion of PrP-res (PrP(Sc)) into contiguous membranes
- PMID: 11867531
- PMCID: PMC125906
- DOI: 10.1093/emboj/21.5.1031
Conversion of raft associated prion protein to the protease-resistant state requires insertion of PrP-res (PrP(Sc)) into contiguous membranes
Abstract
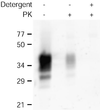
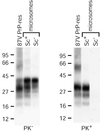
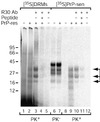
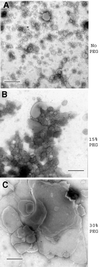
Prion protein (PrP) is usually attached to membranes by a glycosylphosphatidylinositol-anchor that associates with detergent-resistant membranes (DRMs), or rafts. To model the molecular processes that might occur during the initial infection of cells with exogenous transmissible spongiform encephalopathy (TSE) agents, we examined the effect of membrane association on the conversion of the normal protease-sensitive PrP isoform (PrP-sen) to the protease-resistant isoform (PrP-res). A cell-free conversion reaction approximating physiological conditions was used, which contained purified DRMs as a source of PrP-sen and brain microsomes from scrapie-infected mice as a source of PrP-res. Interestingly, DRM-associated PrP-sen was not converted to PrP-res until the PrP-sen was either released from DRMs by treatment with phosphatidylinositol-specific phospholipase C (PI-PLC), or the combined membrane fractions were treated with the membrane-fusing agent polyethylene glycol (PEG). PEG-assisted conversion was optimal at pH 6--7, and acid pre-treating the DRMs was not sufficient to permit conversion without PI-PLC or PEG, arguing against late endosomes/lysosomes as primary compartments for PrP conversion. These observations raise the possibility that generation of new PrP-res during TSE infection requires (i) removal of PrP-sen from target cells; (ii) an exchange of membranes between cells; or (iii) insertion of incoming PrP-res into the raft domains of recipient cells.
Figures





References
-
- Arnold J.E., Tipler,C., Laszlo,L., Hope,J., Landon,M. and Mayer,R.J. (1995) The abnormal isoform of the prion protein accumulates in late-endosome-like organelles in scrapie-infected mouse brain. J. Pathol., 176, 403–411. - PubMed
-
- Batista F.D., Iber,D. and Neuberger,M.S. (2001) B cells acquire antigen from target cells after synapse formation. Nature, 411, 489–494. - PubMed
-
- Bessen R.A., Raymond,G.J. and Caughey,B. (1997) In situ formation of protease-resistant prion protein in transmissible spongiform encephalopathy-infected brain slices. J. Biol. Chem., 272, 15227–15231. - PubMed
-
- Borchelt D.R., Taraboulos,A. and Prusiner,S.B. (1992) Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J. Biol. Chem., 267, 16188–16199. - PubMed
-
- Brown D.A. and Rose,J.K. (1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell, 68, 533–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

