Structure of a functional IGF2R fragment determined from the anomalous scattering of sulfur
- PMID: 11867533
- PMCID: PMC125895
- DOI: 10.1093/emboj/21.5.1054
Structure of a functional IGF2R fragment determined from the anomalous scattering of sulfur
Abstract
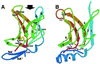
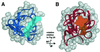
Insulin-like growth factor II receptor (IGF2R) is a multifunctional cell surface receptor implicated in tumour suppression. Its growth inhibitory activity has been associated with an ability to bind IGF-II. IGF2R contains 15 homologous extracellular domains, with domain 11 primarily responsible for IGF-II binding. We report a 1.4 A resolution crystal structure of domain 11, solved using the anomalous scattering signal of sulfur. The structure consists of two crossed beta-sheets forming a flattened beta-barrel. Structural analysis identifies the putative IGF-II binding site at one end of the beta-barrel whilst crystal lattice contacts suggest a model for the full-length IGF2R extracellular region. The structure factors and coordinates of IGF2R domain 11 have been deposited in the Protein Data Bank (accession codes 1GP0 and 1GP3).
Figures




References
-
- Brünger A.T. et al. (1998) Crystallography and NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. - PubMed
-
- Burns J.L. and Hassan,A.B. (2001) Cell survival and proliferation are modified by insulin-like growth factor 2 between days 9 and 10 of mouse gestation. Development, 128, 3819–3830. - PubMed
-
- Byrd J.C., Devi,G.R., de Souza,A.T., Jirtle,R.L. and MacDonald,R.G. (1999) Disruption of ligand binding to the insulin-like growth factor II/mannose 6-phosphate receptor by cancer-associated missense mutations. J. Biol. Chem., 274, 24408–24416. - PubMed
-
- Byrd J.C., Park,J.H., Schaffer,B.S., Garmroudi,F. and MacDonald,R.G. (2000) Dimerization of the insulin-like growth factor II/mannose 6-phosphate receptor. J. Biol. Chem., 275, 18647–18656. - PubMed
-
- Dahms N.M., Rose,P.A., Molkentin,J.D., Zhang,Y. and Brzycki,M.A. (1993) The bovine mannose 6-phosphate/insulin-like growth factor II receptor. The role of arginine residues in mannose 6-phosphate binding. J. Biol. Chem., 268, 5457–5463. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

