The mRNA export factor Dbp5 is associated with Balbiani ring mRNP from gene to cytoplasm
- PMID: 11867546
- PMCID: PMC125910
- DOI: 10.1093/emboj/21.5.1177
The mRNA export factor Dbp5 is associated with Balbiani ring mRNP from gene to cytoplasm
Abstract
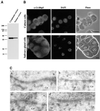
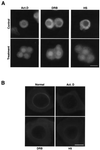
The DEAD box RNA helicase Dbp5 is essential for nucleocytoplasmic transport of mRNA-protein (mRNP) complexes. Dbp5 is present mainly in the cytoplasm and is enriched at the cytoplasmic side of nuclear pore complexes (NPCs), suggesting that it acts in the late part of mRNP export. Here, we visualize the assembly and transport of a specific mRNP particle, the Balbiani ring mRNP in the dipteran Chironomus tentans, and show that a Dbp5 homologue in C.tentans, Ct-Dbp5, binds to pre-mRNP co-transcriptionally and accompanies the mRNP to and through the nuclear pores and into the cytoplasm. We also demonstrate that Ct-Dbp5 accumulates in the nucleus and partly disappears from the NPC when nuclear export of mRNA is inhibited. The fact that Ct-Dbp5 is present along the exiting mRNP fibril extending from the nuclear pore into the cytoplasm supports the view that Ct-Dbp5 is involved in restructuring the mRNP prior to translation. Finally, the addition of the export factor Dbp5 to the growing transcript highlights the importance of the co-transcriptional loading process in determining the fate of mRNA.
Figures




References
-
- Alzhanova-Ericsson A.T., Sun X., Visa,N., Kiseleva,E., Wurtz,T. and Daneholt,B. (1996) A protein of the SR family of splicing factors binds extensively to exonic Balbiani ring pre-mRNA and accompanies the RNA from the gene to the nuclear pore. Genes Dev., 10, 2881–2893. - PubMed
-
- Baurén G. and Wieslander,L. (1994) Splicing of Balbiani ring 1 gene pre-mRNA occurs simultaneously with transcription. Cell, 76, 183–192. - PubMed
-
- Cole C.N. (2000) mRNA export: the long and winding road. Nature Cell Biol., 2, E55–E58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

