Visualization of functionally activated circuitry in the brain
- PMID: 11867719
- PMCID: PMC122505
- DOI: 10.1073/pnas.042701199
Visualization of functionally activated circuitry in the brain
Abstract
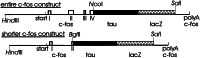
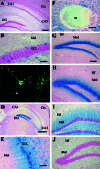
We have used a transgenic approach to visualize functionally activated neurons and their projections. The transgenic mice contain a tau-lacZ fusion gene regulated by the promoter for c-fos, an immediate early gene that is rapidly induced in neurons after functional stimulation. Constitutive expression of beta-galactosidase (beta-gal), the lacZ product, was low and in accord with previous reports of c-fos expression. However, expression of beta-gal in positive neurons was clearly in cell bodies, axons, and dendrites. Treatment of the mice with kainic acid, a strong inducer of c-fos expression, resulted in high induction of beta-gal. beta-gal was induced in the same defined populations of neurons in the brain as those that express c-fos after kainic acid induction. Furthermore, the pattern of beta-gal expression within the neurons changed over time after kainic acid treatment. Early after kainate treatment, beta-gal was found mainly in cell bodies; at later times, expression extended further along the neuronal processes. This expression pattern is consistent with induction and anterograde transport of the Fos-Tau-beta-gal protein in the neurons. To test whether a functionally activated pathway could be visualized, transgenic mice were deprived of water, which activates nuclei involved in body fluid homeostasis. beta-gal induction was traced in neurons and their processes in the lamina terminalis, in magnocellular neurons of the supraoptic and paraventricular nuclei, and in their projections to the posterior pituitary gland. This strategy allowed the mapping of an activated osmoregulatory pathway. This transgenic approach may have general application in the mapping of functionally activated circuitry in the brain.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

