Network of coupled promoting motions in enzyme catalysis
- PMID: 11867722
- PMCID: PMC122427
- DOI: 10.1073/pnas.052005999
Network of coupled promoting motions in enzyme catalysis
Abstract
A network of coupled promoting motions in the enzyme dihydrofolate reductase is identified and characterized. The present identification is based on genomic analysis for sequence conservation, kinetic measurements of multiple mutations, and mixed quantum/classical molecular dynamics simulations of hydride transfer. The motions in this network span time scales of femtoseconds to milliseconds and are found on the exterior of the enzyme as well as in the active site. This type of network has broad implications for an expanded role of the protein fold in catalysis as well as ancillaries such as the engineering of altered protein function and the action of drugs distal to the active site.
Figures


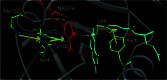

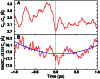
References
-
- Hammes G G. Nature (London) 1964;204:342–343. - PubMed
-
- Kohen A, Cannio R, Bartolucci S, Klinman J P. Nature (London) 1999;399:496–499. - PubMed
-
- Karplus M. J Phys Chem B. 2000;104:11–27.
-
- Osborne M J, Schnell J, Benkovic S J, Dyson H J, Wright P E. Biochemistry. 2001;40:9846–9859. - PubMed
-
- Radkiewicz J L, Brooks C L., III J Am Chem Soc. 2000;122:225–231.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

