Members of the PIAS family act as SUMO ligases for c-Jun and p53 and repress p53 activity
- PMID: 11867732
- PMCID: PMC122440
- DOI: 10.1073/pnas.052559499
Members of the PIAS family act as SUMO ligases for c-Jun and p53 and repress p53 activity
Abstract
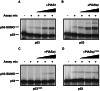
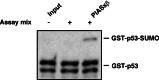
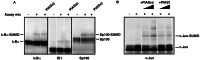
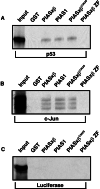
The activity of the p53 tumor suppressor protein and the c-Jun protooncogene is regulated by posttranslational modifications, such as phosphorylation or ubiquitination. In addition, covalent attachment of the ubiquitin-like modifier SUMO appears to modulate their transcriptional activity. Sumoylation proceeds via an enzymatic pathway that is mechanistically analogous to ubiquitination, but requires a different E1-activating enzyme and Ubc9, a SUMO-specific E2-conjugating enzyme. Here, we show that two members of the PIAS family, PIAS1 and PIASxbeta, act as specific E3-like ligases that promote sumoylation of p53 and c-Jun in vitro and in vivo. The PIAS proteins physically interact with both p53 and c-Jun. In addition, they bind to Ubc9, suggesting that they recruit the E2 enzyme to their respective substrate. The SUMO ligase activity requires the conserved zinc-finger domain, which is distantly related to the essential RING-finger motif, found in a subset of ubiquitin ligases. Furthermore, similar to RING-type ubiquitin ligases, PIASxbeta can catalyze its own modification. Hence, these data further extend the analogy between the ubiquitin and SUMO pathway. Strikingly, PIAS proteins strongly repress the transcriptional activity of p53, suggesting that the PIAS-SUMO pathway plays a crucial role in the regulation of p53 and presumably other transcription factors.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

