Ex vivo cell labeling with 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography
- PMID: 11867752
- PMCID: PMC122467
- DOI: 10.1073/pnas.052709599
Ex vivo cell labeling with 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography
Erratum in
-
Correction for Adonai et al., Ex vivo cell labeling with 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography.Proc Natl Acad Sci U S A. 2006 Jul 5;103(27):10526. doi: 10.1073/pnas.0604177103. Epub 2006 Jun 20. Proc Natl Acad Sci U S A. 2006. PMID: 29507149 Free PMC article. No abstract available.
Abstract
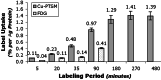
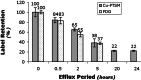
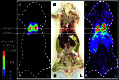
We have used copper-64-pyruvaldehyde-bis(N4-methylthiosemicarbazone) (64Cu-PTSM) to radiolabel cells ex vivo for in vivo positron-emission tomography (PET) imaging studies of cell trafficking in mice and for eventual application in patients. 2-[18F]-Fluoro-2-deoxy-d-glucose (FDG) cell labeling also was evaluated for comparison. 64Cu-PTSM uptake by C6 rat glioma (C6) cells increased for 180 min and then stabilized. The labeling efficiency was directly proportional to 64Cu-PTSM concentration and influenced negatively by serum. Label uptake per cell was greater with 64Cu-PTSM than with FDG. However, both 64Cu-PTSM- and FDG-labeled cells showed efflux of cell activity into supernatant. The 64Cu-PTSM labeling procedure did not interfere significantly with C6 cell viability and proliferation rate. MicroPET images of living mice indicate that tail-vein-injected labeled C6 cells traffic to the lungs and liver. In addition, transient splenic accumulation of radioactivity was clearly detectable in a mouse scanned at 3.33 h postinfusion of 64Cu-PTSM-labeled lymphocytes. In contrast, the liver was the principal organ of tracer localization after tail-vein administration of 64Cu-PTSM alone. These results indicate that in vivo imaging of cell trafficking is possible with 64Cu-PTSM-labeled cells. Given the longer t(1/2) of 64Cu (12.7 h) relative to 18F (110 min), longer cell-tracking periods (up to 24-36 h) should be possible now with PET.
Figures
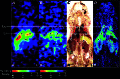
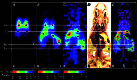
References
-
- Rennen H J, Boerman O C, Oyen W J, Corstens F H. Eur J Nucl Med. 2001;28:241–252. - PubMed
-
- Botti C, Negri D R, Seregni E, Ramakrishna V, Arienti F, Maffioli L, Lombardo C, Bogni A, Pascali C, Crippa F, et al. Eur J Nucl Med. 1997;24:497–504. - PubMed
-
- Melder R J, Elmaleh D, Brownell A L, Brownell G L, Jain R K. J Immunol Methods. 1994;175:79–87. - PubMed
-
- Koike C, Oku N, Watanabe M, Tsukada H, Kakiuchi T, Irimura T, Okada S. Biochim Biophys Acta. 1995;1238:99–106. - PubMed
-
- Koike C, Watanabe M, Oku N, Tsukada H, Irimura T, Okada S. Cancer Res. 1997;57:3612–3619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

