Drug selection with paclitaxel restores expression of linked IL-2 receptor gamma -chain and multidrug resistance (MDR1) transgenes in canine bone marrow
- PMID: 11867757
- PMCID: PMC122483
- DOI: 10.1073/pnas.052712199
Drug selection with paclitaxel restores expression of linked IL-2 receptor gamma -chain and multidrug resistance (MDR1) transgenes in canine bone marrow
Abstract
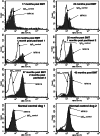
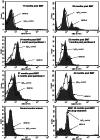
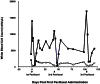
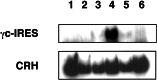
Unstable expression of transferred genes is a major obstacle to successful gene therapy of hematopoietic diseases. We have investigated in a canine large-animal model whether expression of transduced genes can be recovered in vivo. Mixed-breed dogs had undergone autologous bone marrow transplantation (BMT) with stem cell factor and granulocyte-colony-stimulating factor-mobilized retrovirally marked hematopoietic cells. The bicistronic retroviral vector construct allowed for coexpression of MDR1 and human IL-2 receptor common gamma-chain cDNAs. The latter gene is deficient in X-linked severe combined immunodeficiency. After initial high-level expression, P-glycoprotein and the gamma-chain were undetectable in blood and bone marrow 17 months post-BMT. Six months later, one dog was treated i.v. with 125 mg/m2 paclitaxel. Three administrations restored expression of the two linked genes to high levels in blood and bone marrow. Two dogs treated with higher paclitaxel doses died from myelosuppression after the first administration. As determined by flow cytometry, both genes were expressed in granulocytes, monocytes, and lymphocytes of the surviving animal. PCR analysis of DNA from peripheral blood confirmed that the retroviral cDNA was increased after paclitaxel treatment, suggesting enrichment of transduced cells. P-glycoprotein was detectable for more than 1 year after cessation of paclitaxel. Repeated analyses of blood and bone marrow aspirates gave no indication of hematopoietic disturbance after BMT with transduced cells and paclitaxel treatment. In summary, we have shown that with the use of a drug-selectable marker gene, chemotherapy can select for cells that express an otherwise nonselected therapeutic gene in blood and bone marrow.
Figures



Similar articles
-
Paclitaxel chemotherapy after autologous stem-cell transplantation and engraftment of hematopoietic cells transduced with a retrovirus containing the multidrug resistance complementary DNA (MDR1) in metastatic breast cancer patients.Clin Cancer Res. 1999 Jul;5(7):1619-28. Clin Cancer Res. 1999. PMID: 10430060 Clinical Trial.
-
Drug-selected co-expression of P-glycoprotein and gp91 in vivo from an MDR1-bicistronic retrovirus vector Ha-MDR-IRES-gp91.J Gene Med. 2003 May;5(5):366-76. doi: 10.1002/jgm.362. J Gene Med. 2003. PMID: 12731085
-
Retroviral marking of canine bone marrow: long-term, high-level expression of human interleukin-2 receptor common gamma chain in canine lymphocytes.Blood. 1998 Sep 1;92(5):1565-75. Blood. 1998. PMID: 9716584
-
Improved retroviral vectors for hematopoietic stem cell protection and in vivo selection.J Hematother. 1996 Aug;5(4):323-9. doi: 10.1089/scd.1.1996.5.323. J Hematother. 1996. PMID: 8877707 Review.
-
Transfer of the MDR1 (multidrug resistance) gene: protection of hematopoietic cells from cytotoxic chemotherapy, and selection of transduced cells in vivo.Cytokines Mol Ther. 1995 Mar;1(1):11-20. Cytokines Mol Ther. 1995. PMID: 9384659 Review.
Cited by
-
Survival of the fittest: in vivo selection and stem cell gene therapy.Blood. 2006 Mar 1;107(5):1751-60. doi: 10.1182/blood-2005-06-2335. Epub 2005 Nov 3. Blood. 2006. PMID: 16269617 Free PMC article. Review.
-
In vivo selection of hematopoietic progenitor cells and temozolomide dose intensification in rhesus macaques through lentiviral transduction with a drug resistance gene.J Clin Invest. 2009 Jul;119(7):1952-63. doi: 10.1172/JCI37506. Epub 2009 Jun 8. J Clin Invest. 2009. PMID: 19509470 Free PMC article.
-
Towards in vivo amplification: Overcoming hurdles in the use of hematopoietic stem cells in transplantation and gene therapy.World J Stem Cells. 2015 Dec 26;7(11):1233-50. doi: 10.4252/wjsc.v7.i11.1233. World J Stem Cells. 2015. PMID: 26730268 Free PMC article. Review.
-
Topical colchicine selection of keratinocytes transduced with the multidrug resistance gene (MDR1) can sustain and enhance transgene expression in vivo.Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):13096-101. doi: 10.1073/pnas.192247899. Epub 2002 Sep 16. Proc Natl Acad Sci U S A. 2002. PMID: 12235361 Free PMC article.
References
-
- Gottesman M M, Hrycyna C A, Schoenlein P V, Germann U A, Pastan I. Annu Rev Genet. 1995;29:607–649. - PubMed
-
- Ambudkar S V, Dey S, Hrycyna C A, Ramachandra M, Pastan I, Gottesman M M. Annu Rev Pharmacol Toxicol. 1999;39:361–398. - PubMed
-
- Banerjee D, Schweitzer B I, Volkenandt M, Li M X, Waltham M, Mineishi S, Zhao S C, Bertino J R. Gene. 1994;139:269–274. - PubMed
-
- Machiels J P, Govaerts A S, Guillaume T, Bayat B, Feyens A M, Lenoir E, Goeminne J C, Cole S, Deeley R, Caruso M, et al. Hum Gene Ther. 1999;10:801–811. - PubMed
-
- Sorrentino B P, Brandt S J, Bodine D, Gottesman M M, Pastan I, Cline A, Nienhuis A W. Science. 1992;257:99–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

