Inhaled beclomethasone versus budesonide for chronic asthma
- PMID: 11869673
- PMCID: PMC8717128
- DOI: 10.1002/14651858.CD003530
Inhaled beclomethasone versus budesonide for chronic asthma
Abstract
Background: Beclomethasone dipropionate (BDP) and budesonide (BUD) are used widely in the treatment of chronic asthma. The two drugs have different in vitro pharmacokinetic characteristics. It is unclear whether this translates into clinically significant differences in efficacy or safety when treating children and adults with chronic asthma.
Objectives: To assess clinical outcomes in studies which have compared inhaled BDP and BUD in the treatment of chronic asthma.
Search strategy: We searched the Cochrane Airways Group Trial Register (1999) and reference lists of articles. We contacted trialists and pharmaceutical companies for additional studies and searched abstracts of major respiratory society meetings (1997-1999).
Selection criteria: Prospective, randomised trials comparing BDP to BUD in the treatment of chronic asthma. Two reviewers independently assessed articles for inclusion and methodological quality.
Data collection and analysis: One reviewer extracted data; authors were contacted to clarify missing information. Quantitative analyses where undertaken using Review Manager 4.0.3 with Metaview 3.1.
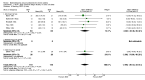
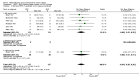
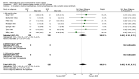
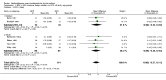
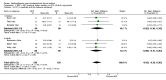
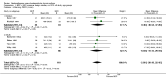
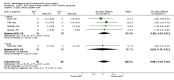
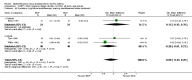
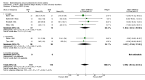
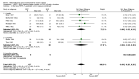
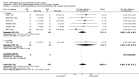
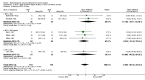
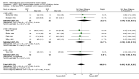
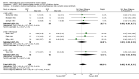
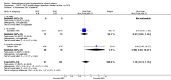
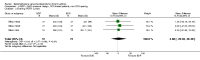
Main results: 24 studies met the criteria for inclusion (1174 subjects). Methodological quality was variable. A meta-analysis of crossover studies did not demonstrate a significant difference between BDP and BUD for FEV1, morning PEF, evening PEF, asthma symptoms or rescue beta2 agonist use, over a dose range of 400 to 1000 mcg/d. The majority of crossover trials had significant design flaws related to a lack of washout and/or failure to exclude carryover effects so the results must be viewed with caution. A single crossover study with adequate washout showed that BUD 400 mcg/d delivered via Turbohaler dry powder inhaler (DPI) may be more effective than BDP 400 mcg/d delivered via Rotahaler DPI in reducing histamine bronchial hyper-responsiveness: Weighted Mean Difference (WMD) 0.43 log10 PC20 FEV1 (95% Confidence Intervals (CI) 0.05, 0.81 log10 PC20 FEV1). A meta-analysis of two parallel group, dose down-titration studies (231 patients) showed that less BUD delivered via a Turbohaler DPI was required to maintain control in adults asthmatics compared to BDP delivered via metered dose inhaler with or without a spacer: WMD 444 mcg/d (95% CI 332, 556 mcg/d).
Reviewer's conclusions: There is limited high quality randomised controlled trial data comparing the relative efficacy of BDP and BUD. Current guidelines (BTS 1997, GINA 1995, NHLBI 1997) assume BDP and BUD to have equal efficacy, such that for each defined level of asthma severity, the recommended doses BDP and BUD are the same. Although there is some data to suggest that BUD via Turbohaler is more effective than BDP via either Rotahaler or MDI (with and without spacer), these comparisons are confounded by use of different delivery devices, and are not sufficient to warrant a change in guideline recommendations.
Conflict of interest statement
None
Figures



































































































































Similar articles
-
Fluticasone versus beclomethasone or budesonide for chronic asthma in adults and children.Cochrane Database Syst Rev. 2007 Oct 17;2007(4):CD002310. doi: 10.1002/14651858.CD002310.pub4. Cochrane Database Syst Rev. 2007. PMID: 17943772 Free PMC article.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.Health Technol Assess. 2008 May;12(19):iii-iv, 1-360. doi: 10.3310/hta12190. Health Technol Assess. 2008. PMID: 18485271
-
Fluticasone versus beclomethasone or budesonide for chronic asthma.Cochrane Database Syst Rev. 2002;(1):CD002310. doi: 10.1002/14651858.CD002310. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2004;(2):CD002310. doi: 10.1002/14651858.CD002310.pub2. PMID: 11869636 Updated.
-
Inhaled fluticasone versus inhaled beclomethasone or inhaled budesonide for chronic asthma in adults and children.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD002310. doi: 10.1002/14651858.CD002310.pub3. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2007 Oct 17;(4):CD002310. doi: 10.1002/14651858.CD002310.pub4. PMID: 15846637 Updated.
-
Inhaled beclomethasone versus placebo for chronic asthma.Cochrane Database Syst Rev. 2000;(4):CD002738. doi: 10.1002/14651858.CD002738. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2005 Jan 25;(1):CD002738. doi: 10.1002/14651858.CD002738.pub2. PMID: 11034752 Updated.
Cited by
-
Biophysical interaction between corticosteroids and natural surfactant preparation: implications for pulmonary drug delivery using surfactant a a carrier.Soft Matter. 2012 Jan 14;8(2):504-511. doi: 10.1039/c1sm06444d. Epub 2011 Nov 1. Soft Matter. 2012. PMID: 28747989 Free PMC article.
-
Development and Practical Application of a Multiple-Criteria Decision Analysis Framework on Respiratory Inhalers: Is It Always Useful in the MOH Malaysia Medicines Formulary Listing Context?MDM Policy Pract. 2021 Mar 30;6(1):2381468321994063. doi: 10.1177/2381468321994063. eCollection 2021 Jan-Jun. MDM Policy Pract. 2021. PMID: 33855190 Free PMC article.
-
Guidelines for diagnosis and management of bronchial asthma: Joint ICS/NCCP (I) recommendations.Lung India. 2015 Apr;32(Suppl 1):S3-S42. doi: 10.4103/0970-2113.154517. Lung India. 2015. PMID: 25948889 Free PMC article. No abstract available.
-
Comparison of clinical effects of beclomethasone dipropionate & budesonide in treatment of children with mild persistent asthma: A double-blind, randomized, controlled study.Indian J Med Res. 2016 Aug;144(2):250-257. doi: 10.4103/0971-5916.195040. Indian J Med Res. 2016. PMID: 27934805 Free PMC article. Clinical Trial.
-
An integrative review of systematic reviews related to the management of breathlessness in respiratory illnesses.BMC Pulm Med. 2010 Dec 9;10:63. doi: 10.1186/1471-2466-10-63. BMC Pulm Med. 2010. PMID: 21143887 Free PMC article. Review.
References
References to studies included in this review
Baran 1987 {published data only}
-
- Baran D. A comparison of inhaled budesonide and beclomethasone dipropionate in childhood asthma. British Journal of Diseases of the Chest 1987;81(2):170‐5. - PubMed
Bisgaard 1988 {published data only}
-
- Bisgaard H, Damkjaer Nielsen M, Andersen B, Andersen P, Foged N, Fuglsang G, et al. Adrenal function in children with bronchial asthma treated with beclomethasone dipropionate or budesonide. Journal of Allergy & Clinical Immunology 1988;81(6):1088‐95. - PubMed
Bjorkander 1982a {published data only}
-
- Bjorkander J, Formgren H, Johansson SA, Millqvist E. Methodological aspects on clinical trials with inhaled corticosteroids: results of two comparisons between two steroid aerosols in patients with asthma. European Journal of Respiratory Diseases ‐ Supplement 1982;122:108‐17. - PubMed
Boe 1989 {published data only}
-
- Boe J, Rosenhall L, Alton M, Carlsson LG, Carlsson U, Hermansson BA, et al. Comparison of dose‐response effects of inhaled beclomethasone dipropionate and budesonide in the management of asthma. Allergy: European Journal of Allergy & Clinical Immunology 1989;44(5):349‐55. - PubMed
Brambilla 1994 {published data only}
-
- Brambilla C, Godard P, Lacronique J, Allaert FA, Duroux P, Blaive B, et al. A 3‐month comparative dose‐reduction study with inhaled beclomethasone dipropionate and budesonide in the management of moderate to severe adult asthma. Drug Investigation 1994;8(1):49‐56.
Dal Negro 1997 {published data only}
-
- Dal Negro R, Micheletto C, Ciani F, Muzzi A, Crimi E, Filippa P, et al. Efficacy and safety of inhaled beclomethasone dipropionate dry powder in the treatment of chronic asthma: A controlled study vs. budesonide. European Respiratory Journal. 1997:351S.
Ebden 1986 {published data only}
Field 1982 {published data only}
Greefhorst 1992 {published data only}
-
- Greefhorst APM. Budesonide and terbutaline delivered via Turbuhaler compared to BDP and salbutamol delivered via Rotahaler. European Respiratory Journal. 1992; Vol. 5, issue Suppl 15:360S.
Hamalainen 1998 {published data only}
-
- Hamalainen KM, Laurikaninen K, Leinonen M, Jager L. Comparison of two multidose powder inhalers (MDPI) in the treatment of asthma with inhaled corticosteroids. European Respiratory Journal. 1998:61S.
Keelan 1984 {published data only}
-
- Keelan P, Gray P, Kelly P, Frame M. Comparison of a new corticosteroid aerosol, budesonide, with beclomethasone dipropionate in the treatment of chronic asthma. Irish Medical Journal 1984;77(8):244‐7. - PubMed
Micheletto 1997 {published data only}
-
- Micheletto C, Mauroner L, Burti E, Turco P, Pomari L, Cantini L, Dal Negro R. Inhaled beclomethasone dipropionate and budesonide dry powder in chronic asthma: Lung function‐serum ECP relationship. European Respiratory Journal. 1997:351S. - PubMed
Nicolaizik 1994 {published data only}
-
- Nicolaizik WH, Marchant JL, Preece MA, Warner JO. Endocrine and lung function in asthmatic children on inhaled corticosteroids. American Journal of Respiratory & Critical Care Medicine 1994;150(3):624‐8. - PubMed
Pedersen 1988 {published data only}
-
- Pedersen S, Fuglsang G. Urine cortisol excretion in children treated with high doses of inhaled corticosteroids: a comparison of budesonide and beclomethasone. European Respiratory Journal 1988;1(5):433‐5. - PubMed
Petrie 1990 {published data only}
-
- Petrie GR, Choo‐Kang YFJ, Clark RA, Milledge JSSPR, Whitfield RJ, Higgins AJ. An assessment of the acceptability of two breath‐actuated corticosteroid inhalers comparison of Turbohaler(TM) with Diskhaler(TM). Drug Investigation 1990;2(2):129‐31.
Rafferty 1985 {published data only}
-
- Rafferty P, Tucker LG, Frame MH, Fergusson RJ, Biggs BA, Crompton GK. Comparison of budesonide and beclomethasone dipropionate in patients with severe chronic asthma: assessment of relative prednisolone‐sparing effects. British Journal of Diseases of the Chest 1985;79(3):244‐50. - PubMed
Selroos 1994 {published data only}
-
- Selroos O, Backman R, Forsen KO, Lofroos AB, Niemisto M, Pietinalho A, et al. Clinical efficacy of budesonide Turbuhaler compared with that of beclomethasone dipropionate pMDI with volumatic spacer. A 2‐year randomized study in 102 asthma patients. Allergy: European Journal of Allergy & Clinical Immunology 1994;49(10):833‐6. - PubMed
Springer 1987 {published data only}
Stiksa 1982a {published data only}
-
- Stiksa G, Glennow C, Johannesson N. A open cross‐over trial with budesonide and beclomethasone dipropionate in patients with bronchial asthma. European Journal of Respiratory Diseases ‐ Supplement 1982;122:266‐7.
Stiksa 1982b {published data only}
-
- Stiksa G, Glennow C, Johannesson N. A open cross‐over trial with budesonide and beclomethasone dipropionate in patients with bronchial asthma. European Journal of Respiratory Diseases ‐ Supplement 1982;122:266‐7.
Stiksa 1985 {published data only}
-
- Stiksa G, Glennow C. Once daily inhalation of budesonide in the treatment of chronic asthma: a clinical comparison. Annals of Allergy 1985;55(1):49‐51. - PubMed
Svendsen 1992 {published data only}
-
- Svendsen UG, Frolund L, Heinig JH, Madsen F, Nielsen NH, Weeke B. High‐dose inhaled steroids in the management of asthma. A comparison of the effects of budesonide and beclomethasone dipropionate on pulmonary function, symptoms, bronchial responsiveness and the adrenal function. Allergy: European Journal of Allergy and Clinical Immunology 1992;47(2 Pt 2):174‐80. - PubMed
-
- Svendsen UG, Frolund L, Heinig JH, Madsen F, Nielsen NH, Weeke B. [High dose inhaled steroids in the treatment of bronchial asthma. A comparison of the effects of budesonide and beclomethasone dipropionate on pulmonary function, symptoms, bronchial reactivity and adrenocortical function]. Ugeskrift for Laeger 1993;155(28):2197‐202. - PubMed
Tjwa 1995 {published data only}
-
- Tjwa MK. Budesonide inhaled via Turbuhaler: a more effective treatment for asthma than beclomethasone dipropionate via Rotahaler. Annals of Allergy, Asthma & Immunology 1995;75(2):107‐11. - PubMed
Willey 1982 {published data only}
-
- Willey RF, Godden DJ, Carmichael J, Preston P, Frame MH, Crompton GK. Twice daily inhalation of a new corticosteroid, budesonide, in the treatment of chronic asthma. European Journal of Respiratory Diseases ‐ Supplement 1982;122:138‐42. - PubMed
References to studies excluded from this review
Birkebaek 1997 {published data only}
-
- Birkebaek NH, Esberg BH, Andersen K, Wolthers OD, Hassager C. [Budesonide and beclomethasone dipropionate inhalation powders. The effect on bone and collagen turnover in children]. Ugeskrift for Laeger 1997;159(17):2559‐62. - PubMed
Bjorkander 1982b {published data only}
-
- Bjorkander J, Formgren H, Johansson SA, Millqvist E. Methodological aspects on clinical trials with inhaled corticosteroids: results of two comparisons between two steroid aerosols in patients with asthma. European Journal of Respiratory Diseases ‐ Supplement 1982;122:108‐17. - PubMed
de Graaff 1996 {published data only}
-
- Graaff CS, Burgh JHAM, Stallaert RALM, Prins J, Lier AA. A double‐blind comparative study of the inhaled corticosteroids budesonide and beclomethasone dipropionate in dry powder inhalers in asthmatic patients. European Respiratory Journal. 1996; Vol. 5, issue Suppl 15:S359‐60.
Kiviranta 1993 {published data only}
Piquet 1996 {published data only}
-
- Piquet J, Zuck P, Dennewald G, Dugue P, Grivaux M, Brun P, et al. Equally efficacious asthma management with budesonide 800 mug administered by Turbuhaler(TM) or with beclomethasone dipropionate >=1500 mug given through a pressurized metered‐dose inhaler with spacer. Advances in Therapy 1996;13(1):38‐50. - PubMed
Rosenhall 1982 {published data only}
-
- Rosenhall L, Lundqvist G, Adelroth E, Glennow C. Comparison between inhaled and oral corticosteroids in patients with chronic asthma. European Journal of Respiratory Diseases ‐ Supplement 1982;122:154‐62. - PubMed
Struijs 1997 {published data only}
-
- Struijs A, Mulder H. The effects of inhaled glucocorticoids on bone mass and biochemical markers of bone homeostasis: a 1‐year study of beclomethasone versus budesonide. Netherlands Journal of Medicine 1997;50(6):233‐7. - PubMed
References to studies awaiting assessment
Baxter‐Jones 2000 {published data only}
-
- Baxter‐Jones AD, Helms PJ. Early introduction of inhaled steroids in wheezing children presenting in primary care. A pilot study. Clinical & Experimental Allergy 2000;30(11):1618‐26. - PubMed
Brand 2001 {published data only}
-
- Brand PL, Baan‐Slootweg OH, Heynens JW, Vries TW, Versteegh FG, Vreuls RC, et al. Comparison of handling and acceptability of two spacer devices in young children with asthma. Acta Paediatrica 2001;90(2):133‐6. - PubMed
Dal Negro 1999b {published data only}
-
- Dal Negro R, Micheletto C, Tognella S, Mauroner L, Burti E, Turco P, et al. Effect of inhaled beclomethasone dipropionate and budesonide dry powder on pulmonary function and serum eosinophil cationic protein in adult asthmatics. Journal of Investigational Allergology & Clinical Immunology 1999;9(4):241‐7. - PubMed
Hernandez 1995 {published data only}
-
- Hernandez J, Garcia‐Selles FJ, Negro JM, Pascual A, Sola J, Miralles JC, et al. [Comparative study] of beclomethasone and budesonide, with same posology (400 micrograms every 12 hours), in the control of cortico‐ dependent intrinsic asthma]. Allergologia et Immunopathologia 1995;23(5):193‐201. - PubMed
Jager 2000 {published data only}
-
- Jager L, Laurikainen K, Leinonen M, Silvasti M. Beclomethasone dipropionate Easyhaler is as effective as budesonide Turbohaler in the control of asthma and is preferred by patients. International Journal of Clinical Practice 2000;54(6):368‐72. - PubMed
Kraszko 1999 {published data only}
-
- Kraszko P, Vondra D, Malolepszy J, Svensson M, Baly J, Fiserova J, et al. Budesonide via Turbuhaler, 400 mug daily, is as effective as beclomethasone dipropionate via pressurised MDI, 800 mug daily, for control of mild to moderate asthmatic patients. Journal of Clinical Research 1999;2:47‐55.
Lee 1995 {published data only}
-
- Lee YC, Rhee YK. Effect of inhaled steroids on the cortisol concentration by different dosage or delivery method. Tuberculosis & Respiratory Diseases 1995;42(6):888‐99.
Reichel 2001 {published data only}
-
- Reichel W, Dahl R, Ringdal N, Zetterstrom O, Elshout FJ, Laitinen LA. Extrafine beclomethasone dipropionate breath‐actuated inhaler (400 micrograms/day) versus budesonide dry powder inhaler (800 micrograms/day) in asthma. International Journal of Clinical Practice 2001;55(2):100‐6. - PubMed
Additional references
Boobis 1998
-
- Boobis AR. Comparative physiochemical and pharmacokinetic profiles of inhaled beclomethasone dipropionate and budesonide. Respiratory Medicine 1998;92(Suppl B):2‐6. - PubMed
BTS 1997
-
- British Thoracic Society. The British guidelines on asthma management 1995 review and position statement. Thorax 1997;52(Suppl 1):S1‐20.
GINA 1995
-
- National Asthma Education and Prevention Program. Global strategy for asthma management and prevention NHBLI/WHO workshop report. Bethesda MD: NIH/National Heart, Lung and Blood Institute 1995, issue Publication No. 95‐3659.
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. - PubMed
Johansson 1982
-
- Johansson SA, Andersson KE, Brattsand R, Gruvstad E, Hedner P. Topical and systemic glucocorticoid potencies of budesonide and beclomethasone dipropionate in man. European Journal of Clinical Pharmacology 1982;22:523‐9. - PubMed
NHLBI 1997
-
- National Asthma Education and Prevention Program. Guidelines for the Diagnosis and Managment of Asthma, Expert Panel Report No. 2. Bethesda MD: NIH/National Heart, Lung and Blood Institute 1997, issue NIH Publication No. 97‐4051.
Pethica 1998
Stellato 1999
-
- Stellato C, Atsuta J, Bickel CA, Schleimer RP. An in vitro comparison of commonly used topical glucocorticoid preparations. Journal of Allergy & Clinical Immunology 1999;104(3 Pt 1):623‐9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

