Preoperative induction chemotherapy with cisplatin and irinotecan for pathological N(2) non-small cell lung cancer
- PMID: 11870532
- PMCID: PMC2375275
- DOI: 10.1038/sj.bjc.6600117
Preoperative induction chemotherapy with cisplatin and irinotecan for pathological N(2) non-small cell lung cancer
Abstract
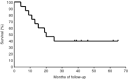
We conducted a phase I/II study to investigate whether the surgical resection after induction chemotherapy with cisplatin and irinotecan was feasible and could improve the treatment outcome for patients with pathological N(2) non-small cell lung cancer. Fifteen patients with stage IIIA non-small cell lung cancer having mediastinal lymph node metastases proved by mediastinoscopy were eligible. Both cisplatin (60 mg m(-2)) and irinotecan (50 mg m(-2)) were given on days 1 and 8. Patients received two cycles of chemotherapy after 3-4 weeks interval. Induction was followed by surgical resection in 4-6 weeks. Patients who had documented tumour regression after preoperative chemotherapy received two additional cycles of chemotherapy and other patients received radiotherapy postoperatively. After the induction chemotherapy, the objective response rate was 73%. All the 15 patients received surgical resection and complete resection was achieved in 11 (73%) patients. There was no operation-related death and one death due to radiation pneumonitis during postoperative radiotherapy. The median time from entry to final analysis was 46.5 months, ranging from 22 to 68 months. The 5-year survival rate was 40% for all the 15 patients and it was 55% for the 11 patients who underwent complete resection. We conclude that the surgical resection after induction chemotherapy with cisplatin and irinotecan is feasible, and associated with low morbidity and high respectability.
Figures
Similar articles
-
Induction chemoradiotherapy is superior to induction chemotherapy for the survival of non-small-cell lung cancer patients with pathological mediastinal lymph node metastasis.Interact Cardiovasc Thorac Surg. 2012 Dec;15(6):954-60. doi: 10.1093/icvts/ivs412. Epub 2012 Sep 12. Interact Cardiovasc Thorac Surg. 2012. PMID: 22976995 Free PMC article.
-
A phase II study of cisplatin and irinotecan as induction chemotherapy followed by concomitant thoracic radiotherapy with weekly low-dose irinotecan in unresectable, stage III, non-small cell lung cancer: JCOG 9706.Jpn J Clin Oncol. 2011 Jan;41(1):25-31. doi: 10.1093/jjco/hyq163. Epub 2010 Aug 27. Jpn J Clin Oncol. 2011. PMID: 20802006 Clinical Trial.
-
Phase II study of irinotecan plus cisplatin induction followed by concurrent twice-daily thoracic irradiation with etoposide plus cisplatin chemotherapy for limited-disease small-cell lung cancer.J Clin Oncol. 2005 May 20;23(15):3488-94. doi: 10.1200/JCO.2005.01.082. J Clin Oncol. 2005. PMID: 15908658 Clinical Trial.
-
Establishment of the standard regimen for non-small-cell lung cancer in Japan.Oncology (Williston Park). 2001 Jan;15(1 Suppl 1):13-8. Oncology (Williston Park). 2001. PMID: 11221016 Review.
-
Pulmonary resection after curative intent radiotherapy (>59 Gy) and concurrent chemotherapy in non-small-cell lung cancer.Ann Thorac Surg. 2004 Oct;78(4):1200-5; discussion 1206. doi: 10.1016/j.athoracsur.2004.04.085. Ann Thorac Surg. 2004. PMID: 15464470 Review.
Cited by
-
Neoadjuvant immunotherapy and neoadjuvant chemotherapy in resectable non-small cell lung cancer: A systematic review and single-arm meta-analysis.Front Oncol. 2022 Sep 21;12:901494. doi: 10.3389/fonc.2022.901494. eCollection 2022. Front Oncol. 2022. PMID: 36212419 Free PMC article.
-
Induction chemoradiotherapy followed by surgical resection for clinical T3 or T4 locally advanced non-small cell lung cancer.Ann Surg Oncol. 2012 Aug;19(8):2685-92. doi: 10.1245/s10434-012-2302-x. Epub 2012 Mar 7. Ann Surg Oncol. 2012. PMID: 22396006 Free PMC article.
-
Induction chemoradiotherapy is superior to induction chemotherapy for the survival of non-small-cell lung cancer patients with pathological mediastinal lymph node metastasis.Interact Cardiovasc Thorac Surg. 2012 Dec;15(6):954-60. doi: 10.1093/icvts/ivs412. Epub 2012 Sep 12. Interact Cardiovasc Thorac Surg. 2012. PMID: 22976995 Free PMC article.
-
Preoperative concurrent chemoradiotherapy with cisplatin and docetaxel in patients with locally advanced non-small-cell lung cancer.Br J Cancer. 2004 Mar 8;90(5):979-84. doi: 10.1038/sj.bjc.6601624. Br J Cancer. 2004. PMID: 14997193 Free PMC article.
-
Phase I/II study of docetaxel and cisplatin with concurrent thoracic radiation therapy for locally advanced non-small-cell lung cancer.Br J Cancer. 2003 Sep 1;89(5):795-802. doi: 10.1038/sj.bjc.6601217. Br J Cancer. 2003. PMID: 12942107 Free PMC article. Clinical Trial.
References
-
- AlbainKSRuschVWCrowleyJJRiceTWTurrisiIIIATWeickJKLonchynaVAPresantCAMcKennaRJGandaraDR1995Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stage IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805 J Clin Oncol 1318801892 - PubMed
-
- FowlerWCLangerCJCurranWJKellerSM1993Postoperative complications after combined neoadjuvant treatment of lung cancer Ann Thorac Surg 55986989 - PubMed
-
- HsiangY-HLiuLF1988Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin Cancer Res 4817221726 - PubMed
-
- MathisenDJWainJCWrightCChoiNCareyRHilgenbergAGrossbardMLynchTGrilloH1996Assessment of preoperative accelerated radiotherapy and chemotherapy in stage IIIA (N2) non-small-cell lung cancer J Thorac Cardiovasc Surg 111123133 - PubMed
-
- OkenMMDavisTEGreechRHMcFaddenETTormeyDCCarbonePPHortonJ1982Toxicity and response criteria of the Eastern Cooperative Oncology Group Am J Clin Oncol 5649655 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical