Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases
- PMID: 11872087
- PMCID: PMC1782650
- DOI: 10.1046/j.1365-2567.2002.01345.x
Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases
Abstract
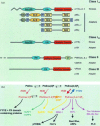
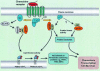
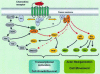
The role of chemokines in mediating directional cell migration is well established, but more recently it has become evident that chemokines are able to couple to distinct signalling pathways that are involved in not only chemotaxis, but also cell growth and transcriptional activation. The signalling pathway controlled by the phosphoinositide 3-kinase (PI3K) family of lipid kinases has been the focus of much attention with respect to their role in chemokine-mediated functional responses. Indeed, there now exists convincing biochemical, pharmacological and genetic evidence that both CC and CXC chemokines stimulate PI3K-dependent chemotaxis of inflammatory cells such as eosinophils, macrophages, neutrophils and T lymphocytes. This review considers the role of individual PI3Ks (e.g. the p85/p110 heterodimer, PI3Kgamma and PI3KC2alpha) as well their downstream effector targets in mediating chemokine-stimulated cell migration.
Figures
Comment in
-
Chemokines--linking receptors to response.Immunology. 2002 Feb;105(2):121-4. Immunology. 2002. PMID: 11872086 Free PMC article. Review. No abstract available.
References
-
- Rollins BJ. Chemokines. Blood. 1997;90:909–28. - PubMed
-
- Von Andrian U, Mackay CR. T-cell function and migration. N Engl J Med. 2001;343:1020–34. - PubMed
-
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–8. - PubMed
-
- Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

