Age-dependent increase of peritoneal B-1b B cells in SCID mice
- PMID: 11872095
- PMCID: PMC1782648
- DOI: 10.1046/j.1365-2567.2002.01360.x
Age-dependent increase of peritoneal B-1b B cells in SCID mice
Abstract
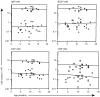
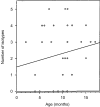
The impact of increasing age upon immunoglobulin production and B-lymphocyte generation in "leaky" severe combined immune-defective (SCID) mice was examined by enzyme-linked immunosorbent assay and flow cytometry. By 1 year of age, the mice had normal numbers of B cells in their peritoneal cavity, while their spleen had very few immunoglobulin M-positive (IgM+) cells. The majority of B cells expressed the CD11b marker characteristic of the B-1b subset. B-1a (CD5+) cells were present at a lower frequency and B-2 cells were absent. The frequency of mice producing detectable immunoglobulin increased with age, and isotype diversity within individual mice was variable. IgM production was most frequently observed followed by IgG3 and IgG2a, then IgG1, and finally IgA. The selective persistence of the B-1 B-cell subset in the peritoneal cavity of aging SCID mice is a natural model for the study of those genetic and environmental influences that determine lymphocyte longevity.
Figures




References
-
- Bosma G, Custer R, Bosma M. A severe combined immunodeficiency in the mouse. Nature. 1983;310:527–30. - PubMed
-
- Fulop G, Phillips R. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990;347:479–82. - PubMed
-
- Blunt T, Finnie N, Taccioli G, et al. Defective DNA-dependent protein kinase activity is linked to V (D) J recombination and DNA repair defects associated with murine scid mutation. Cell. 1995;80:813–23. - PubMed
-
- Carroll A, Bosma M. Detection and characterization of functional T cells in mice with severe combined immune deficiency. Eur J Immunol. 1988;18:1965–71. - PubMed
-
- Carroll A, Hardy R, Bosma M. Occurrence of mature B (IgM+,B220+) and T (CD3+) lymphocytes in SCID mice. J Immunol. 1989;143:1087–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

