Low molecular mass dinitrosyl nonheme-iron complexes up-regulate noradrenaline release in the rat tail artery
- PMID: 11872148
- PMCID: PMC65697
- DOI: 10.1186/1471-2210-2-3
Low molecular mass dinitrosyl nonheme-iron complexes up-regulate noradrenaline release in the rat tail artery
Abstract
Background: Dinitrosyl nonheme-iron complexes can appear in cells and tissues overproducing nitric oxide. It is believed that due to their chemical nature these species may be implicated in certain pathophysiological events. We studied the possible role of low molecular mass dinitrosyl iron complexes in the control of noradrenaline release in electrically stimulated rat tail artery.
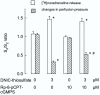
Results: A model complex, dinitrosyl-iron-thiosulfate (at 1-10 microM) produced a concentration-dependent enhancement of electrical field stimulated [3H]noradrenaline release (up to 2 fold). At the same time, dinitrosyl-iron-thiosulfate inhibited neurogenic vasoconstriction, consistent with its nitric oxide donor properties. A specific inhibitor of cyclic GMP dependent protein kinase, Rp-8pCPT-cGMPS, partially inhibited the effect of dinitrosyl-iron-thiosulfate on neurogenic vasoconstriction, but not on [3H]noradrenaline release. Another model complex, dinitrosyl-iron-cysteine (at 3 microM) elicited similar responses as dinitrosyl-iron-thiosulfate. Conventional NO and NO+ donors such as sodium nitroprusside, S-nitroso-L-cysteine or S-nitroso-glutathione (at 10 microM) had no effect on [3H]noradrenaline release, though they potently decreased electrically-induced vasoconstriction. The "false complex", iron(II)-thiosulfate showed no activity.
Conclusions: Low molecular mass iron dinitrosyl complexes can up-regulate the stimulation-evoked release of vascular [3H]noradrenaline, apparently independently of their NO donor properties. This finding may have important implications in inflammatory tissues.
Figures

Similar articles
-
Role of the L-arginine-NO pathway and of cyclic GMP in electrical field-induced noradrenaline release and vasoconstriction in the rat tail artery.Br J Pharmacol. 1992 Dec;107(4):976-82. doi: 10.1111/j.1476-5381.1992.tb13394.x. Br J Pharmacol. 1992. PMID: 1334757 Free PMC article.
-
Inhibition of cyclic GMP-dependent protein kinase-mediated effects by (Rp)-8-bromo-PET-cyclic GMPS.Br J Pharmacol. 1995 Dec;116(8):3110-6. doi: 10.1111/j.1476-5381.1995.tb15112.x. Br J Pharmacol. 1995. PMID: 8719784 Free PMC article.
-
Inhibition of arterial contraction by dinitrosyl-iron complexes: critical role of the thiol ligand in determining rate of nitric oxide (NO) release and formation of releasable NO stores by S-nitrosation.Biochem Pharmacol. 2003 Dec 15;66(12):2365-74. doi: 10.1016/j.bcp.2003.07.017. Biochem Pharmacol. 2003. PMID: 14637194
-
Effects of cyclic AMP and analogues on neurogenic transmission in the rat tail artery.Br J Pharmacol. 1994 Feb;111(2):625-31. doi: 10.1111/j.1476-5381.1994.tb14782.x. Br J Pharmacol. 1994. PMID: 8004406 Free PMC article.
-
[Prospect of using magnetic nanoparticles for potentiating the anticarcinogenic action of dinitrosyl iron complexes with thiol-containing ligands].Biofizika. 2011 Sep-Oct;56(5):868-72. Biofizika. 2011. PMID: 22117444 Review. Russian.
Cited by
-
Iron nitrosyl complexes as models for biological nitric oxide transfer reagents.J Biol Inorg Chem. 2006 Apr;11(3):359-70. doi: 10.1007/s00775-006-0084-y. Epub 2006 Mar 7. J Biol Inorg Chem. 2006. PMID: 16520978
References
-
- Henry Y, Lepoivre M, Drapier JC, Ducrocq C, Boucher JL, Guissani A. EPR characterization of molecular targets for NO in mammalian cells and organelles. FASEB J. 1993;7:1124–1134. - PubMed
-
- Kleschyov AL, Muller B, Stoeckel ME, Stoclet JC. Adventitia-derived nitric oxide in rat aortas exposed to endotoxin: cell origin and functional consequences. Am J Physiol heart circ physiol. 2000;279:H2743–H2751. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

