Use of ultramolecular potencies of allergen to treat asthmatic people allergic to house dust mite: double blind randomised controlled clinical trial
- PMID: 11872551
- PMCID: PMC67767
- DOI: 10.1136/bmj.324.7336.520
Use of ultramolecular potencies of allergen to treat asthmatic people allergic to house dust mite: double blind randomised controlled clinical trial
Abstract
Objective: To evaluate the efficacy of homoeopathic immunotherapy on lung function and respiratory symptoms in asthmatic people allergic to house dust mite.
Design: Double blind randomised controlled trial.
Setting: 38 general practices in Hampshire and Dorset.
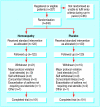
Participants: 242 people with asthma and positive results to skin prick test for house dust mite; 202 completed clinic based assessments, and 186 completed diary based assessments.
Intervention: After a four week baseline assessment, participants were randomised to receive oral homoeopathic immunotherapy or placebo and then assessed over 16 weeks with three clinic visits and diary assessments every other week.
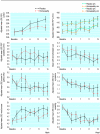
Outcome measure: Clinic based assessments: forced expiratory volume in one second (FEV(1)), quality of life, and mood. DIARY BASED ASSESSMENTS: morning and evening peak expiratory flow, visual analogue scale of severity of asthma, quality of life, and daily mood.
Results: There was no difference in most outcomes between placebo and homoeopathic immunotherapy. There was a different pattern of change over the trial for three of the diary assessments: morning peak expiratory flow (P=0.025), visual analogue scale (P=0.017), and mood (P=0.035). At week three there was significant deterioration for visual analogue scale (P=0.047) and mood (P=0.013) in the homoeopathic immunotherapy group compared with the placebo group. Any improvement in participants' asthma was independent of belief in complementary medicine.
Conclusion: Homoeopathic immunotherapy is not effective in the treatment of patients with asthma. The different patterns of change between homoeopathic immunotherapy and placebo over the course of the study are unexplained.
Figures
Comment in
-
Randomised controlled trials for homoeopathy.BMJ. 2002 Mar 2;324(7336):498-9. doi: 10.1136/bmj.324.7336.498. BMJ. 2002. PMID: 11872535 Free PMC article. No abstract available.
-
Randomised controlled trials for homoeopathy. Studies comparing homoeopathy and placebo are unhelpful.BMJ. 2002 Jul 6;325(7354):41; author reply 41. BMJ. 2002. PMID: 12102071 No abstract available.
-
Randomised controlled trials for homoeopathy. Language is being distorted.BMJ. 2002 Jul 6;325(7354):41; author reply 41. BMJ. 2002. PMID: 12102072 No abstract available.
-
Randomised controlled trials for homoeopathy. Study is in effect trying to compare apples with oranges.BMJ. 2002 Jul 6;325(7354):41; author reply 41. BMJ. 2002. PMID: 12102073 No abstract available.
-
Randomised controlled trials for homoeopathy. When is useful improvement a waste of time? Double positive paradox of negative trials.BMJ. 2002 Jul 6;325(7354):41; author reply 41. BMJ. 2002. PMID: 12102074 No abstract available.
Summary for patients in
-
Homeopathy ineffective for asthma.J Fam Pract. 2002 Jul;51(7):602. J Fam Pract. 2002. PMID: 12160495 No abstract available.
References
-
- National Association of Health Authorities and Trusts. Complementary therapies in the NHS. Birmingham: NAHAT; 1993. (research paper 10).
-
- Ernst E. Use of complementary therapies in childhood asthma. Pediatr Asthma Allergy Immunol. 1998;12:29–32.
-
- Letts M. The jury's out. National Asthma Campaign report. London: National Asthma Campaign; 1998.
-
- Eizayaga FX, Eizayage J, Eizayaga F. Homoeopathic treatment of bronchial asthma. Br Homeopath J. 1996;85:28–33.
