Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction
- PMID: 11874240
- PMCID: PMC1435380
- DOI: 10.1359/jbmr.2002.17.3.493
Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction
Abstract
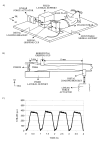
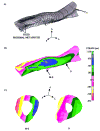
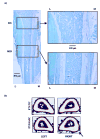
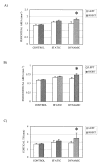
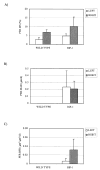
Transgenic and knockout mice present a unique opportunity to study mechanotransduction pathways in vivo, but the difficulty inherent with applying externally controlled loads to the small mouse skeleton has hampered this approach. We have developed a novel device that enables the noninvasive application of controlled mechanical loads to the murine tibia. Calibration of tissue strains induced by the device indicated that the normal strain environment was repeatable across loading bouts. Two in vivo studies were performed to show the usefulness of the device. Using C57Bl/6J mice, we found that dynamic but not static loading increased cortical bone area. This result is consistent with previous models of bone adaptation, and the lack of adaptation induced by static loading serves as a negative control for the device. In a preliminary study, transgenic mice selectively overexpressing insulin-like growth factor 1 (IGF-1) in osteoblasts underwent a low-magnitude loading regimen. Periosteal bone formation was elevated 5-fold in the IGF-1-overexpressing mice but was not elevated in wild-type littermates, showing the potential for synergism between mechanical loading and selected factors. Based on these data, we anticipate that the murine tibia-loading device will enhance assessment of mechanotransduction pathways in vivo and, as a result, has the potential to facilitate novel gene discovery and optimization of synergies between drug therapies and mechanical loading.
Conflict of interest statement
The authors have no conflict of interest.
Figures

References
-
- Riggs BL, Melton LJ. Involutional osteoporosis. N Engl J Med. 1986;314:1676–1686. - PubMed
-
- Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. - PubMed
-
- Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriquez-Portales J, Downs RW, Dequeker J, Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III osteoporosis treatment study group. N Engl J Med. 1995;333:1437–1443. - PubMed
-
- NIAMS 2000 Bone biology and bone diseases research: Strategic plan (FY 2000 –2004). www.nih.gov/niams/strategicplan/bone.htm
-
- Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37:411–417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

