Maximizing the value of medicines by including pharmacogenetic research in drug development and surveillance
- PMID: 11874384
- PMCID: PMC1874316
- DOI: 10.1046/j.0306-5251.2001.01556.x
Maximizing the value of medicines by including pharmacogenetic research in drug development and surveillance
Abstract
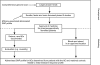
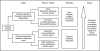
Genetics provides significant opportunities to maximize the safety and efficacy of medicines. Over the next 3--5 years, it may be possible to develop tools that use selective information from patients' DNA to enable healthcare professionals to predict more accurately those patients at risk of serious adverse events to some medicines currently available. This is likely to be followed, over the next 5--10 years, by the application of the technology to predict more accurately if individual patients will obtain a therapeutic benefit from a particular medicine. The ability to accurately predict patient response will inevitably change the way medicines are developed, evaluated, and prescribed. Advances in single nucleotide polymorphism (SNP) map technology are likely to drive this innovation. Abbreviated SNP profiles will provide the means to define medicine response tests, thereby allowing clinicians to select the medicine to which the patient is likely to gain the greatest benefit and least risk. This will help to maximize efficacy and reduce the incidence of drug-related adverse events. It may be possible to identify SNP profiles during larger Phase II clinical trials which predict efficacy, and use these to form the basis of Phase III entry criteria. As a result, Phase III trials may be streamlined for many medicines making them smaller, more efficient, and more focused. In addition it may be possible to incorporate pharmacogenetics into postmarketing surveillance strategies to provide a means to identify SNPs which predict uncommon serious adverse drug reactions, and so refine the initial medicine response test. The ability to develop drugs with a predictable response will allow clinicians to provide targeted treatment for patients, with greater confidence of safety and efficacy. Patients therefore will receive more efficacious, timely, and well-tolerated medicines. The challenge for those involved in drug development is to model and evaluate the application of pharmacogenetics so that steps can be taken to realize this potential.
Figures
Similar articles
-
Idiosyncratic reactions to drugs: can medicine response profiles provide a dynamic drug surveillance system?Clin Chem Lab Med. 2000 Sep;38(9):815-8. doi: 10.1515/CCLM.2000.117. Clin Chem Lab Med. 2000. PMID: 11097333
-
The medical and economic roles of pipeline pharmacogenetics: Alzheimer's disease as a model of efficacy and HLA-B(*)5701 as a model of safety.Neuropsychopharmacology. 2009 Jan;34(1):6-17. doi: 10.1038/npp.2008.153. Epub 2008 Oct 15. Neuropsychopharmacology. 2009. PMID: 18923406 Review.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Pharmacogenetics.Hum Mol Genet. 2001 Oct 1;10(20):2261-7. doi: 10.1093/hmg/10.20.2261. Hum Mol Genet. 2001. PMID: 11673409 Review.
-
[Pharmacogenetics--implications for health management and health care economics].Med Klin (Munich). 2002 Jul 15;97(7):420-8. doi: 10.1007/s00063-002-1177-1. Med Klin (Munich). 2002. PMID: 12168482 German.
Cited by
-
Genotyping of drug targets: a method to predict adverse drug reactions?Drug Saf. 2002;25(8):553-60. doi: 10.2165/00002018-200225080-00002. Drug Saf. 2002. PMID: 12113641 Review.
-
Linking pharmacovigilance with pharmacogenetics.Drug Saf. 2004;27(15):1171-84. doi: 10.2165/00002018-200427150-00002. Drug Saf. 2004. PMID: 15588114
-
Clinical pharmacology: special safety considerations in drug development and pharmacovigilance.Drug Saf. 2004;27(8):535-54. doi: 10.2165/00002018-200427080-00006. Drug Saf. 2004. PMID: 15154826 Review.
-
Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages.Milbank Q. 2004;82(4):661-87. doi: 10.1111/j.0887-378X.2004.00327.x. Milbank Q. 2004. PMID: 15595946 Free PMC article.
References
-
- Anderson WH, Fitzgerald CQ, Manasco PK. Current and future applications of pharmacogenomics. New Horizons. 1999;7:262–269.
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions on hospitalized patients. A meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. - PubMed
-
- Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost of illness model. Arch Intern Med. 1995;155:1949–1956. - PubMed
-
- Cohen LJ, Devane CL. Clinical implications of antidepressant pharmacokinetics and pharmacogenetics. Ann Pharmacother. 1996;3:1471–1480. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials