Tumour necrosis factor (TNF) in psoriatic arthritis: pathophysiology and treatment with TNF inhibitors
- PMID: 11874829
- PMCID: PMC1754073
- DOI: 10.1136/ard.61.4.298
Tumour necrosis factor (TNF) in psoriatic arthritis: pathophysiology and treatment with TNF inhibitors
Abstract
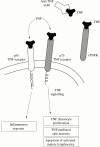
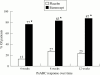
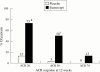
High levels of proinflammatory cytokines, including tumour necrosis factor (TNF), have been detected in psoriatic skin lesions and joints of patients with the inflammatory disease. Early results of treatment of psoriatic arthritis and psoriasis with TNF neutralising agents are encouraging, but whether these agents will be able to improve long term outcomes, such as disability, is not yet known.
Figures


Similar articles
-
TNFalpha therapy in psoriatic arthritis and psoriasis.Ann Rheum Dis. 2004 Jul;63(7):755-8. doi: 10.1136/ard.2004.020719. Ann Rheum Dis. 2004. PMID: 15194567 Free PMC article. No abstract available.
-
Cytokine blockers in psoriatic arthritis.Ann Rheum Dis. 2001 Nov;60 Suppl 3(Suppl 3):iii37-40. doi: 10.1136/ard.60.90003.iii37. Ann Rheum Dis. 2001. PMID: 11890650 Free PMC article. Clinical Trial.
-
TNF alpha inhibitors in the treatment of psoriasis and psoriatic arthritis.BioDrugs. 2005;19(1):47-57. doi: 10.2165/00063030-200519010-00006. BioDrugs. 2005. PMID: 15691217 Review.
-
TNFalpha inhibition in psoriatic arthritis: cause for hope.Joint Bone Spine. 2001 Mar;68(2):106-8. doi: 10.1016/s1297-319x(01)00257-3. Joint Bone Spine. 2001. PMID: 11324924 Review. No abstract available.
-
[Biological agents in treatment of psoriatic arthritis].Pol Merkur Lekarski. 2008 Jul;25(145):97-100. Pol Merkur Lekarski. 2008. PMID: 18839626 Review. Polish.
Cited by
-
Altered bone remodeling in psoriatic arthritis.Curr Rheumatol Rep. 2008 Aug;10(4):311-7. doi: 10.1007/s11926-008-0050-5. Curr Rheumatol Rep. 2008. PMID: 18662512 Free PMC article. Review.
-
The comparison of efficacy of different imaging techniques (conventional radiography, ultrasonography, magnetic resonance) in assessment of wrist joints and metacarpophalangeal joints in patients with psoriatic arthritis.Pol J Radiol. 2013 Jan;78(1):18-29. doi: 10.12659/PJR.883764. Pol J Radiol. 2013. PMID: 23494635 Free PMC article.
-
The Role of Bone Morphogenetic Protein Receptor Type 2 (BMPR2) and the Prospects of Utilizing Induced Pluripotent Stem Cells (iPSCs) in Pulmonary Arterial Hypertension Disease Modeling.Cells. 2022 Nov 29;11(23):3823. doi: 10.3390/cells11233823. Cells. 2022. PMID: 36497082 Free PMC article. Review.
-
Heterogeneity of autoimmune diseases: pathophysiologic insights from genetics and implications for new therapies.Nat Med. 2015 Jul;21(7):730-8. doi: 10.1038/nm.3897. Epub 2015 Jun 29. Nat Med. 2015. PMID: 26121193 Free PMC article. Review.
-
Outcome measures in psoriatic arthritis.Curr Rheumatol Rep. 2005 Jun;7(3):195-200. doi: 10.1007/s11926-996-0039-x. Curr Rheumatol Rep. 2005. PMID: 15918995 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

