Secretory pathway of trypanosomatid parasites
- PMID: 11875130
- PMCID: PMC120783
- DOI: 10.1128/MMBR.66.1.122-154.2002
Secretory pathway of trypanosomatid parasites
Abstract
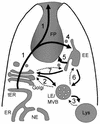
The Trypanosomatidae comprise a large group of parasitic protozoa, some of which cause important diseases in humans. These include Trypanosoma brucei (the causative agent of African sleeping sickness and nagana in cattle), Trypanosoma cruzi (the causative agent of Chagas' disease in Central and South America), and Leishmania spp. (the causative agent of visceral and [muco]cutaneous leishmaniasis throughout the tropics and subtropics). The cell surfaces of these parasites are covered in complex protein- or carbohydrate-rich coats that are required for parasite survival and infectivity in their respective insect vectors and mammalian hosts. These molecules are assembled in the secretory pathway. Recent advances in the genetic manipulation of these parasites as well as progress with the parasite genome projects has greatly advanced our understanding of processes that underlie secretory transport in trypanosomatids. This article provides an overview of the organization of the trypanosomatid secretory pathway and connections that exist with endocytic organelles and multiple lytic and storage vacuoles. A number of the molecular components that are required for vesicular transport have been identified, as have some of the sorting signals that direct proteins to the cell surface or organelles in the endosome-vacuole system. Finally, the subcellular organization of the major glycosylation pathways in these parasites is reviewed. Studies on these highly divergent eukaryotes provide important insights into the molecular processes underlying secretory transport that arose very early in eukaryotic evolution. They also reveal unusual or novel aspects of secretory transport and protein glycosylation that may be exploited in developing new antiparasite drugs.
Figures





References
-
- Ace, T. A., S. Gokool, D. McGhie, S. Stager, and D. F. Smith. 1999. Expression of hydrophilic surface proteins in infective stages of Leishmania donovani. Mol. Biochem. Parasitol. 102:191-196. - PubMed
-
- Achleitner, G., B. Gaigg, A. Krasser, E. Kainersdorfer, S. D. Kohlwein, A. Perktold, G. Zellnig, and G. Daum. 1999. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contacts. Eur. J. Biochem. 264:545-553. - PubMed
-
- Acosta Serrano, A., S. Schenkman, N. Yoshida, A. Mehlert, J. M. Richardson, and M. A. J. Ferguson. 1995. The lipid structure of the glycosylphosphatidylinositol-anchored mucin-like sialic acid acceptors of Trypanosoma cruzi changes during parasite differentiation from epimastigotes to infective metacyclic trypomastigote forms. J. Biol. Chem. 270:27244-27253. - PubMed
-
- Acosta-Serrano, A., I. C. Almeida, L. H. Freitas-Junior, N. Yoshida, and S. Schenkman. 2001. The mucin-like glycoprotein super-family of Trypanosoma cruzi: structure and biological roles. Mol. Biochem. Parasitol. 114:143-150. - PubMed
-
- Acosta-Serrano, A., R. N. Cole, and P. T. Englund. 2000. Killing of Trypanosoma brucei by concanavalin A: structural basis of resistance in glycosylation mutants. J. Mol. Biol. 304:633-644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

