Expression and functional activity of CXCR-4 and CCR-5 chemokine receptors in human thymocytes
- PMID: 11876757
- PMCID: PMC1906330
- DOI: 10.1046/j.1365-2249.2002.01775.x
Expression and functional activity of CXCR-4 and CCR-5 chemokine receptors in human thymocytes
Abstract
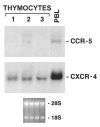
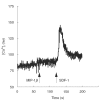
In this paper we addressed the expression of the HIV co-receptors CXCR-4 and CCR-5 in human thymocytes by phenotypic, molecular and functional approaches. Cytofluorimetric analysis disclosed that CXCR-4 was constitutively expressed by freshly isolated thymocytes (~10 000 molecules/cell in about 30% of thymocytes); the receptor was endowed with functional activity, as it mediated polarization, migration and intracellular Ca2+ increase in response to its ligand, SDF-1. On the contrary, CCR-5 expression in freshly isolated thymocytes was significantly lower (<4000 molecules/cell in less than 5% of the cells), and no functional response to CCR-5 agonists could be documented. Northern blot analysis of freshly isolated thymocytes showed high CXCR-4 mRNA levels, whereas the message for CCR-5 was barely detectable. On the other hand, a modest increase in the expression of CCR-5 was associated with in vitro thymocyte stimulation, and CCR-5 density at the cell surface attained CXCR-4 figures in most cases. None the less, no functional response to CCR-5 agonists could be documented in in vitro stimulated thymocytes. In vitro infection of thymocytes by CAT-expressing recombinant HIV bearing the envelope glycoproteins from different isolates showed that T-tropic strains, which use CXCR-4 as a co-receptor, were more efficient in infecting thymocytes than M-tropic strains, which preferentially use CCR-5. Altogether, these data indicate that expression of the major co-receptors involved in infection by M-tropic HIV strains is very poor in human thymocytes, and would suggest that thymocyte infection by M-tropic HIV strains may be a rare event in vivo.
Figures
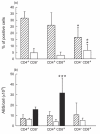
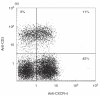


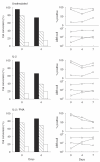
 ), and of the M-tropic Ba-L (□) isolates. The CAT assay was performed as detailed in Materials and methods; data from one of three representative experiments are presented. Right panels: non-infected thymocytes were analysed in parallel for percentage expression and surface density (ABS/cell) of CD4 (Δ), CXCR-4 (○) and CCR-5 (□).
), and of the M-tropic Ba-L (□) isolates. The CAT assay was performed as detailed in Materials and methods; data from one of three representative experiments are presented. Right panels: non-infected thymocytes were analysed in parallel for percentage expression and surface density (ABS/cell) of CD4 (Δ), CXCR-4 (○) and CCR-5 (□).Similar articles
-
CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection.J Immunol. 1998 Sep 15;161(6):3103-13. J Immunol. 1998. PMID: 9743377
-
Isolated human astrocytes are not susceptible to infection by M- and T-tropic HIV-1 strains despite functional expression of the chemokine receptors CCR5 and CXCR4.Glia. 2001 May;34(3):165-77. Glia. 2001. PMID: 11329179
-
Increased replication of T-cell-tropic HIV strains and CXC-chemokine receptor-4 induction in T cells treated with macrophage inflammatory protein (MIP)-1alpha, MIP-1beta and RANTES beta-chemokines.AIDS. 1998 Jan 22;12(2):183-90. doi: 10.1097/00002030-199802000-00008. AIDS. 1998. PMID: 9468367
-
Co-receptors for HIV-1 entry.Curr Opin Immunol. 1997 Aug;9(4):551-62. doi: 10.1016/s0952-7915(97)80110-0. Curr Opin Immunol. 1997. PMID: 9287172 Review.
-
[Chemokines and defense-system cell homing].J Soc Biol. 2001;195(1):9-12. J Soc Biol. 2001. PMID: 11530508 Review. French.
Cited by
-
Impacts of humanized mouse models on the investigation of HIV-1 infection: illuminating the roles of viral accessory proteins in vivo.Viruses. 2015 Mar 23;7(3):1373-90. doi: 10.3390/v7031373. Viruses. 2015. PMID: 25807049 Free PMC article. Review.
-
Bioinformatic analysis of HIV-1 entry and pathogenesis.Curr HIV Res. 2014;12(2):132-61. doi: 10.2174/1570162x12666140526121746. Curr HIV Res. 2014. PMID: 24862329 Free PMC article. Review.
-
T cell specific adapter protein (TSAd) interacts with Tec kinase ITK to promote CXCL12 induced migration of human and murine T cells.PLoS One. 2010 Mar 18;5(3):e9761. doi: 10.1371/journal.pone.0009761. PLoS One. 2010. PMID: 20305788 Free PMC article.
-
Resting CD4+ T lymphocytes but not thymocytes provide a latent viral reservoir in a simian immunodeficiency virus-Macaca nemestrina model of human immunodeficiency virus type 1-infected patients on highly active antiretroviral therapy.J Virol. 2003 Apr;77(8):4938-49. doi: 10.1128/jvi.77.8.4938-4949.2003. J Virol. 2003. PMID: 12663799 Free PMC article.
-
HIV-1 target cells in the CNS.J Neurovirol. 2015 Jun;21(3):276-89. doi: 10.1007/s13365-014-0287-x. Epub 2014 Sep 19. J Neurovirol. 2015. PMID: 25236812 Free PMC article. Review.
References
-
- Alkhatib G, Combadiere C, Broder CC, et al. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–8. - PubMed
-
- Choe H, Farzan M, Sun Y, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–48. - PubMed
-
- Deng H, Liu R, Ellmeier W, et al. Identification of a major co- receptor for primary isolates of HIV-1. Nature. 1996;381:661–6. - PubMed
-
- Doranz BJ, Rucker J, Yi Y, et al. A dual-tropic primary HIV-1 isolate that uses Fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–58. - PubMed
-
- Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

