Mechanisms of action of proteinase-activated receptor agonists on human platelets
- PMID: 11877318
- PMCID: PMC1573223
- DOI: 10.1038/sj.bjp.0704559
Mechanisms of action of proteinase-activated receptor agonists on human platelets
Abstract
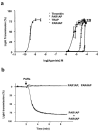
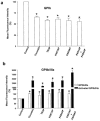
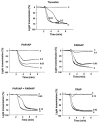
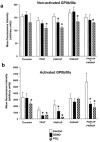
1. We studied the activation of human platelets by thrombin and proteinase activated receptor (PAR)-activating peptides (PAR-APs) [SFLLRNPNDKYEPF-amide (TRAP), TFLLR-amide (PAR1AP) and AYPGKF-amide (PAR4AP)]. 2. PAR agonist-induced platelet aggregation, glycoprotein (GP) Ib and GPIIb/IIIa surface expression and ADP release were measured by light aggregometry, flow cytometry and chemiluminescence. 3. Aggregation inhibitors, including prostacyclin (PGI(2)), nitric oxide-releasing agent (S-nitroso-glutathione, GSNO), aspirin, apyrase, and phenanthroline were used to study the susceptibility of PAR agonist-induced aggregation to pharmacological inhibition. 4. Thrombin was the most potent platelet agonist, followed by PAR1AP, TRAP and PAR4AP. 5. The aggregatory potencies of PAR-APs were not modified by the aminopeptidase inhibitor, amastatin. 6. Subthreshold concentrations of PAR1AP potentiated the effects of PAR4AP to stimulate maximal aggregation. 7. Both PGI(2) and GSNO reduced PAR agonist-induced aggregation and diminished GPIIb/IIIa up-regulation. 8. PAR agonist-induced aggregation was aspirin-insensitive indicating a minor role for TXA(2). 9. In contrast, phenanthroline and apyrase significantly enhanced the anti-aggregatory effects of aspirin against thrombin-, PAR1AP- and TRAP-induced aggregation suggesting the involvement of ADP- and MMP-2-dependent pathways. 10. PAR4AP-induced aggregation (but not PAR1AP-induced aggregation) was entirely ADP-dependent (abolished by apyrase) and resistant to phenanthroline (MMP-2-independent). 11. Thus, the mechanisms of PAR1 and 4-induced platelet aggregation are distinct and depend differentially on their ability to interact with pathways of aggregation, along with the subsequent activation of GPIIb/IIIa receptors.
Figures

Similar articles
-
Platelet-leukocyte aggregation induced by PAR agonists: regulation by nitric oxide and matrix metalloproteinases.Br J Pharmacol. 2004 Dec;143(7):845-55. doi: 10.1038/sj.bjp.0705997. Epub 2004 Nov 8. Br J Pharmacol. 2004. PMID: 15533889 Free PMC article.
-
Protease-activated receptors 1 and 4 do not stimulate G(i) signaling pathways in the absence of secreted ADP and cause human platelet aggregation independently of G(i) signaling.Blood. 2002 May 15;99(10):3629-36. doi: 10.1182/blood.v99.10.3629. Blood. 2002. PMID: 11986217
-
The ATP-gated P2X1 receptor plays a pivotal role in activation of aspirin-treated platelets by thrombin and epinephrine.J Biol Chem. 2008 Jul 4;283(27):18493-504. doi: 10.1074/jbc.M800358200. Epub 2008 May 14. J Biol Chem. 2008. PMID: 18480058
-
Challenges and promises of developing thrombin receptor antagonists.Recent Pat Cardiovasc Drug Discov. 2010 Nov;5(3):162-70. doi: 10.2174/157489010793351980. Recent Pat Cardiovasc Drug Discov. 2010. PMID: 20874673 Review.
-
Thrombin receptor antagonists; recent advances in PAR-1 antagonist development.Curr Med Chem. 2002 Jul;9(13):1229-50. doi: 10.2174/0929867023369934. Curr Med Chem. 2002. PMID: 12052165 Review.
Cited by
-
Inhibition of MCF-7 breast cancer cell-induced platelet aggregation using a combination of antiplatelet drugs.Oncol Lett. 2013 Feb;5(2):675-680. doi: 10.3892/ol.2012.1074. Epub 2012 Dec 13. Oncol Lett. 2013. PMID: 23420392 Free PMC article.
-
Aspirin and P2Y12 inhibition attenuate platelet-induced ovarian cancer cell invasion.BMC Cancer. 2015 Sep 9;15:627. doi: 10.1186/s12885-015-1634-x. BMC Cancer. 2015. PMID: 26353776 Free PMC article.
-
Targeting Platelets for the Treatment of Cancer.Cancers (Basel). 2017 Jul 22;9(7):94. doi: 10.3390/cancers9070094. Cancers (Basel). 2017. PMID: 28737696 Free PMC article. Review.
-
The Ratio of ADP- to TRAP-Induced Platelet Aggregation Quantifies P2Y12-Dependent Platelet Inhibition Independently of the Platelet Count.PLoS One. 2016 Feb 17;11(2):e0149053. doi: 10.1371/journal.pone.0149053. eCollection 2016. PLoS One. 2016. PMID: 26885820 Free PMC article.
-
Tumor Cell-Induced Platelet Aggregation as an Emerging Therapeutic Target for Cancer Therapy.Front Oncol. 2022 Jun 23;12:909767. doi: 10.3389/fonc.2022.909767. eCollection 2022. Front Oncol. 2022. PMID: 35814405 Free PMC article. Review.
References
-
- BLACKHART B.D., EMILSSON K., NGUYEN D., TENG W., MARTELLI A.J., NYSTEDT S., SUNDELIN J., SCARBOROUGH R.M. Ligand cross-reactivity within the proteinase-activated receptor family. J. Biol. Chem. 1996;271:16466–16471. - PubMed
-
- BLOMBACK B. Fibrinogen and fibrin-proteins with complex roles in hemostasis and thrombosis. Thromb. Res. 1996;83:1–75. - PubMed
-
- BORN G.V. Effects of adenosine diphosphate (ADP) and related substances on the adhesiveness of platelets in vitro and in vivo. Br. J. Haematol. 1966;12:37–38. - PubMed
-
- CHEN J., ISHII M., WANG L., ISHII K., COUGHLIN S.R. Thrombin receptor activation: confirmation of the intramolecular tethered liganding hypothesis and discovery of an alternative intermolecular liganding mode. J. Biol.Chem. 1994;268:16041–16045. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

