High RhoA activity maintains the undifferentiated mesenchymal cell phenotype, whereas RhoA down-regulation by laminin-2 induces smooth muscle myogenesis
- PMID: 11877460
- PMCID: PMC2173321
- DOI: 10.1083/jcb.200107049
High RhoA activity maintains the undifferentiated mesenchymal cell phenotype, whereas RhoA down-regulation by laminin-2 induces smooth muscle myogenesis
Abstract
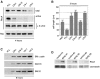
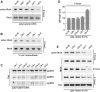
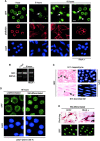
Round embryonic mesenchymal cells have the potential to differentiate into smooth muscle (SM) cells upon spreading/elongation (Yang, Y., K.C. Palmer, N. Relan, C. Diglio, and L. Schuger. 1998. Development. 125:2621-2629; Yang, Y., N.K. Relan, D.A. Przywara, and L. Schuger. 1999. Development. 126:3027-3033; Yang, Y., S. Beqaj, P. Kemp, I. Ariel, and L. Schuger. 2000. J. Clin. Invest. 106:1321-1330). In the developing lung, this process is stimulated by peribronchial accumulation of laminin (LN)-2 (Relan, N.K., Y. Yang, S. Beqaj, J.H. Miner, and L. Schuger. 1999. J. Cell Biol. 147:1341-1350). Here we show that LN-2 stimulates bronchial myogenesis by down-regulating RhoA activity. Immunohistochemistry, immunoblotting, and reverse transcriptase-PCR indicated that RhoA, a small GTPase signaling protein, is abundant in undifferentiated embryonic mesenchymal cells and that its levels decrease along with SM myogenesis. Functional studies using agonists and antagonists of RhoA activation and dominant positive and negative plasmid constructs demonstrated that high RhoA activity was required to maintain the round undifferentiated mesenchymal cell phenotype. This was in part achieved by restricting the localization of the myogenic transcription factor serum response factor (SRF) mostly to the mesenchymal cell cytoplasm. Upon spreading on LN-2 but not on other main components of the extracellular matrix, the activity and level of RhoA decreased rapidly, resulting in translocation of SRF to the nucleus. Both cell elongation and SRF translocation were prevented by overexpression of dominant positive RhoA. Once the cells underwent SM differentiation, up-regulation of RhoA activity induced rather than inhibited SM gene expression. Therefore, our studies suggest a novel mechanism whereby LN-2 and RhoA modulate SM myogenesis.
Figures



Similar articles
-
Stretch-induced alternative splicing of serum response factor promotes bronchial myogenesis and is defective in lung hypoplasia.J Clin Invest. 2000 Dec;106(11):1321-30. doi: 10.1172/JCI8893. J Clin Invest. 2000. PMID: 11104785 Free PMC article.
-
Cell elongation induces laminin alpha2 chain expression in mouse embryonic mesenchymal cells: role in visceral myogenesis.J Cell Biol. 1999 Dec 13;147(6):1341-50. doi: 10.1083/jcb.147.6.1341. J Cell Biol. 1999. PMID: 10601345 Free PMC article.
-
Heterogeneous nuclear ribonucleoprotein-H plays a suppressive role in visceral myogenesis.Mech Dev. 2001 Jun;104(1-2):79-87. doi: 10.1016/s0925-4773(01)00377-x. Mech Dev. 2001. PMID: 11404082
-
The FAKs about blood vessel assembly.Circ Res. 2003 Feb 21;92(3):255-7. doi: 10.1161/01.res.0000059260.91342.6e. Circ Res. 2003. PMID: 12595334 Review. No abstract available.
-
Embryological origin of airway smooth muscle.Proc Am Thorac Soc. 2008 Jan 1;5(1):4-10. doi: 10.1513/pats.200704-049VS. Proc Am Thorac Soc. 2008. PMID: 18094078 Free PMC article. Review.
Cited by
-
The extracellular matrix in development and morphogenesis: a dynamic view.Dev Biol. 2010 May 1;341(1):126-40. doi: 10.1016/j.ydbio.2009.10.026. Epub 2009 Oct 23. Dev Biol. 2010. PMID: 19854168 Free PMC article. Review.
-
P311 induces a TGF-beta1-independent, nonfibrogenic myofibroblast phenotype.J Clin Invest. 2002 Nov;110(9):1349-58. doi: 10.1172/JCI15614. J Clin Invest. 2002. PMID: 12417574 Free PMC article.
-
Sustained Activation of Rho GTPases Promotes a Synthetic Pulmonary Artery Smooth Muscle Cell Phenotype in Neprilysin Null Mice.Arterioscler Thromb Vasc Biol. 2018 Jan;38(1):154-163. doi: 10.1161/ATVBAHA.117.310207. Epub 2017 Nov 30. Arterioscler Thromb Vasc Biol. 2018. PMID: 29191928 Free PMC article.
-
Calcium phosphate surfaces promote osteogenic differentiation of mesenchymal stem cells.J Cell Mol Med. 2008 Jan-Feb;12(1):281-91. doi: 10.1111/j.1582-4934.2007.00103.x. J Cell Mol Med. 2008. PMID: 18366455 Free PMC article.
-
Motility, survival, and proliferation.Compr Physiol. 2012 Jan;2(1):255-81. doi: 10.1002/cphy.c110018. Compr Physiol. 2012. PMID: 23728975 Free PMC article. Review.
References
-
- Belaguli, N.S., L.A. Schildmeyer, and R.J. Schwartz. 1997. Organization and myogenic restricted expression of the murine serum response factor gene. A role for autoregulation. J. Biol. Chem. 272:18222–18231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

