Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis
- PMID: 11877461
- PMCID: PMC2173317
- DOI: 10.1083/jcb.200108123
Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis
Abstract
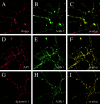
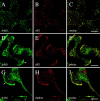
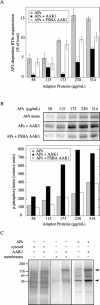
The mu 2 subunit of the AP2 complex is known to be phosphorylated in vitro by a copurifying kinase, and it has been demonstrated recently that mu 2 phosphorylation is required for transferrin endocytosis (Olusanya, O., P.D. Andrews, J.R. Swedlow, and E. Smythe. 2001. Curr. Biol. 11:896-900). However, the identity of the endogenous kinase responsible for this phosphorylation is unknown. Here we identify and characterize a novel member of the Prk/Ark family of serine/threonine kinases, adaptor-associated kinase (AAK)1. We find that AAK1 copurifies with adaptor protein (AP)2 and that it directly binds the ear domain of alpha-adaptin in vivo and in vitro. In neuronal cells, AAK1 is enriched at presynaptic terminals, whereas in nonneuronal cells it colocalizes with clathrin and AP2 in clathrin-coated pits and at the leading edge of migrating cells. AAK1 specifically phosphorylates the mu subunit in vitro, and stage-specific assays for endocytosis show that mu phosphorylation by AAK1 results in a decrease in AP2-stimulated transferrin internalization. Together, these results provide strong evidence that AAK1 is the endogenous mu 2 kinase and plays a regulatory role in clathrin-mediated endocytosis. These results also lend support to the idea that clathrin-mediated endocytosis is controlled by cycles of phosphorylation/desphosphorylation.
Figures





References
-
- Bar-Zvi, D., and D. Branton. 1986. Clathrin-coated vesicles contain two protein kinase activities. Phosphorylation of clathrin beta-light chain by casein kinase II. J. Biol. Chem. 261:9614–9621. - PubMed
-
- Bauerfeind, R., K. Takei, and P. De Camilli. 1997. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. J. Biol. Chem. 272:30984–30992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

