Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis
- PMID: 11877474
- PMCID: PMC150889
- DOI: 10.1172/JCI13572
Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis
Abstract
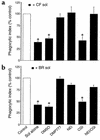
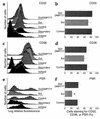
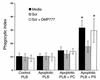
Cystic fibrosis is characterized by an early and sustained influx of inflammatory cells into the airways and by release of proteases. Resolution of inflammation is normally associated with the orderly removal of dying apoptotic inflammatory cells through cell recognition receptors, such as the phosphatidylserine receptor, CD36, and alpha v integrins. Accordingly, removal of apoptotic inflammatory cells may be impaired in persistent inflammatory responses such as that seen in cystic fibrosis airways. Examination of sputa from cystic fibrosis and non-cystic fibrosis bronchiectasis patients demonstrated an abundance of apoptotic cells, in excess of that seen in patients with chronic bronchitis. In vitro, cystic fibrosis and bronchiectasis airway fluid directly inhibited apoptotic cell removal by alveolar macrophages in a neutrophil elastase-dependent manner, suggesting that elastase may impair apoptotic cell clearance in vivo. Flow cytometry demonstrated that neutrophil elastase cleaved the phosphatidylserine receptor, but not CD36 or CD32 (Fc gamma RII). Cleavage of the phosphatidylserine receptor by neutrophil elastase specifically disrupted phagocytosis of apoptotic cells, implying a potential mechanism for delayed apoptotic cell clearance in vivo. Therefore, defective airway clearance of apoptotic cells in cystic fibrosis and bronchiectasis may be due to elastase-mediated cleavage of phosphatidylserine receptor on phagocytes and may contribute to ongoing airway inflammation.
Figures
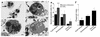
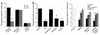
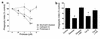

References
-
- Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. - PubMed
-
- Lethem MI, James SL, Marriott C, Burke JF. The origin of DNA associated with mucus glycoproteins in cystic fibrosis sputum. Eur Respir J. 1990;3:19–23. - PubMed
-
- McDonald PP, Fadok VA, Bratton D, Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J Immunol. 1999;163:6164–6172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

