Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining
- PMID: 11877485
- PMCID: PMC2193772
- DOI: 10.1084/jem.20011786
Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining
Abstract
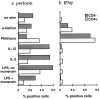
CD1d-restricted natural killer (NK)T cells are known to potently secrete T helper (Th)1 and Th2 cytokines and to mediate cytolysis, but it is unclear how these contrasting functional activities are regulated. Using lipid antigen-loaded CD1d tetramers, we have distinguished two subsets of CD1d-restricted T cells in fresh peripheral blood that differ in cytokine production and cytotoxic activation. One subset, which was CD4(-), selectively produced the Th1 cytokines interferon gamma and tumor necrosis factor alpha, and expressed NKG2d, a marker associated with cytolysis of microbially infected and neoplastic cells. This subset up-regulated perforin after exposure to interleukin (IL)-2 or IL-12. In contrast, CD4(+) CD1d-restricted NKT cells potently produced both Th1 and Th2 cytokines, up-regulated perforin in response to stimulation by phorbol myristate acetate and ionomycin but not IL-2 or IL-12, and could be induced to express CD95L. Further, for both CD1d-restricted NKT cell subsets, we found that antigenic stimulation induced cytokine production but not perforin expression, whereas exposure to inflammatory factors enhanced perforin expression but did not stimulate cytokine production. These results show that the various activities of CD1d-restricted T cells in tumor rejection, autoimmune disease, and microbial infections could result from activation of functionally distinct subsets, and that inflammatory and antigenic stimuli may influence different effector functions.
Figures



References
-
- Porcelli, S.A., and R.L. Modlin. 1999. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17:297–329. - PubMed
-
- Bendelac, A., O. Lantz, M.E. Quimby, J.W. Yewdell, J.R. Bennink, and R.R. Brutkiewicz. 1995. CD1 recognition by mouse NK1+ T lymphocytes. Science. 268:863–865. - PubMed
-
- Chen, H., and W.E. Paul. 1997. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J. Immunol. 159:2240–2249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

