Distinct functional lineages of human V(alpha)24 natural killer T cells
- PMID: 11877486
- PMCID: PMC2193771
- DOI: 10.1084/jem.20011908
Distinct functional lineages of human V(alpha)24 natural killer T cells
Abstract
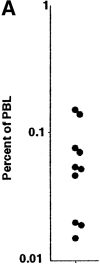
CD1d-restricted autoreactive natural killer (NK)T cells have been reported to regulate a range of disease conditions, including type I diabetes and immune rejection of cancer, through the secretion of either T helper (Th)2 or Th1 cytokines. However, mechanisms underlying Th2 versus Th1 cytokine secretion by these cells are not well understood. Since most healthy subjects express <1 NKT cell per 1,000 peripheral blood lymphocytes (PBLs), we devised a new method based on the combined used of T cell receptor (TCR)-specific reagents alpha-galactosylceramide (alphaGalCer) loaded CD1d-tetramers and anti-V(alpha)24 monoclonal antibody, to specifically identify and characterize these rare cells in fresh PBLs. We report here that CD4(+) and CD4(-)CD8(-) (double negative [DN]) NKT cell subsets represent functionally distinct lineages with marked differences in their profile of cytokine secretion and pattern of expression of chemokine receptors, integrins, and NK receptors. CD4(+) NKT cells were the exclusive producers of interleukin (IL)-4 and IL-13 upon primary stimulation, whereas DN NKT cells had a strict Th1 profile and prominently expressed several NK lineage receptors. These findings may explain how NKT cells could promote Th2 responses in some conditions and Th1 in others, and should be taken into consideration for intervention in relevant diseases.
Figures







References
-
- Park, S.H., and A. Bendelac. 2000. CD1-restricted T-cell responses and microbial infection. Nature. 406:788–792. - PubMed
-
- Godfrey, D.I., K.J.L. Hammond, L.D. Poulton, and A.G. Baxter. 2000. NKT cell facts, functions and fallacies. Immunol. Today. 21:573–583. - PubMed
-
- Janeway, C.A., Jr. 1990. γδ T cells: research on the frontlines of defence. Res. Immunol. 141:688–695. - PubMed
-
- Benlagha, K., and A. Bendelac. 2000. CD1d-restricted mouse Vα14 and human Vα24 T cells: lymphocytes of innate immunity. Semin. Immunol. 12:537–542. - PubMed
-
- Hammond, K.J.L., L.D. Poulton, L.J. Palmisano, P.A. Silveira, D.I. Godfrey, and A.G. Baxter. 1998. α/β-T cell receptor (TCR)+CD4−CD8− (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J. Exp. Med. 187:1047–1056. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

