Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus
- PMID: 11877488
- PMCID: PMC2193766
- DOI: 10.1084/jem.20012023
Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus
Abstract
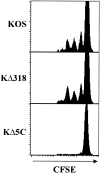
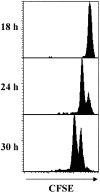
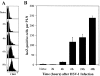
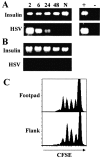
Localized cutaneous herpes simplex virus type 1 (HSV-1) infection leads to arming and initial expansion of cytotoxic T lymphocytes (CTLs) in the draining popliteal lymph nodes (PLNs) followed by migration and further proliferation in the spleen. To accurately characterize the sequence of events involved in the activation and generation of anti-HSV CTLs, we used T cell receptor (TCR) transgenic mice specific for the immunodominant epitope from HSV glycoprotein B (gB(498-505)). We describe the detection of the initiation of antigen presentation in the draining lymph nodes by 4-6 h after infection with HSV-1. Analysis of CD69 up-regulation revealed activation of gB-specific CD8(+) T cells by 6-8 h after infection. Furthermore, we show that T cell proliferation begins no sooner than 24 h after activation and is marked by the concurrent appearance of CTL activity in the PLNs. These events are not dependent on the presence of virus in the draining lymph nodes, and suggest a requirement for recruitment of professional antigen-presenting cells to the site of T cell activation. Consequently, we have defined the initiation of the CD8(+) T cell-mediated response to cutaneous HSV-1 infection, demonstrating that the immune response to localized viral infection depends only on the appearance of cells presenting virus-derived antigen and commences with remarkable swiftness.
Figures

References
-
- Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. - PubMed
-
- Cose, S.C., C.M. Jones, M.E. Wallace, W.R. Heath, and F.R. Carbone. 1997. Antigen-specific CD8+ T cell subset distribution in lymph nodes draining the site of herpes simplex virus infection. Eur. J. Immunol. 27:2310–2316. - PubMed
-
- McNally, J.M., D. Dempsey, R.M. Wolcott, R. Chervenak, and S.R. Jennings. 1999. Phenotypic identification of antigen-dependent and antigen-independent CD8 CTL precursors in the draining lymph node during acute cutaneous herpes simplex virus type 1 infection. J. Immunol. 163:675–681. - PubMed
-
- Coles, R.M., S.N. Mueller, W.R. Heath, F.R. Carbone, and A.G. Brooks. 2002. Progression of armed CTL from draining lymph node to spleen shortly after localised infection with HSV-1. J. Immunol. 168:834–838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

