Estimating the precursor frequency of naive antigen-specific CD8 T cells
- PMID: 11877489
- PMCID: PMC2193761
- DOI: 10.1084/jem.20001021
Estimating the precursor frequency of naive antigen-specific CD8 T cells
Abstract
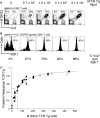
The constraint of fitting a diverse repertoire of antigen specificities in a limited total population of lymphocytes results in the frequency of naive cells specific for any given antigen (defined as the precursor frequency) being below the limit of detection by direct measurement. We have estimated this precursor frequency by titrating a known quantity of antigen-specific cells into naive recipients. Adoptive transfer of naive antigen-specific T cell receptor transgenic cells into syngeneic nontransgenic recipients, followed by stimulation with specific antigen, results in activation and expansion of both donor and endogenous antigen-specific cells in a dose-dependent manner. The precursor frequency is equal to the number of transferred cells when the transgenic and endogenous responses are of equal magnitude. Using this method we have estimated the precursor frequency of naive CD8 T cells specific for the H-2D(b)-restricted GP33-41 epitope of LCMV to be 1 in 2 x 10(5). Thus, in an uninfected mouse containing approximately 2-4 x 10(7) naive CD8 T cells we estimate there to be 100-200 epitope-specific cells. After LCMV infection these 100-200 GP33-specific naive CD8 T cells divide >14 times in 1 wk to reach a total of approximately 10(7) cells. Approximately 5% of these activated GP33-specific effector CD8 T cells survive to generate a memory pool consisting of approximately 5 x 10(5) cells. Thus, an acute LCMV infection results in a >1,000-fold increase in precursor frequency of D(b)GP33-specific CD8 T cells from 2 x 10(2) naive cells in uninfected mice to 5 x 10(5) memory cells in immunized mice.
Figures



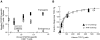
References
-
- Givan, A.L., J.L Fisher, M. Waugh, M.S. Ernstoff and P.K. Wallace. 1999. A flow cytometric method to estimate the precursor frequencies of cells proliferating in response to specific antigens. J. Immunol. Methods 230:99-112. - PubMed
-
- Murali-Krishna, K., L.L. Lau, S. Sambhara, F. Lemonnier, J.D. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 286:1377–1381. - PubMed
-
- Murali-Krishna, K., J.D. Altman, M. Suresh, D.J. Sourdive, A.J. Zajac, J.D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 8:177–187. - PubMed
-
- Blattman, J.N., D.J.D. Sourdive, K. Murali-Krishna, R. Ahmed, and J.D. Altman. 2000. Evolution of the T cell repertoire during primary, memory, and recall responses to viral infection. J. Immunol. 165:6081–6090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

