Collagen of articular cartilage
- PMID: 11879535
- PMCID: PMC128915
- DOI: 10.1186/ar380
Collagen of articular cartilage
Abstract
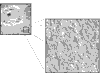
The extracellular framework and two-thirds of the dry mass of adult articular cartilage are polymeric collagen. Type II collagen is the principal molecular component in mammals, but collagens III, VI, IX, X, XI, XII and XIV all contribute to the mature matrix. In developing cartilage, the core fibrillar network is a cross-linked copolymer of collagens II, IX and XI. The functions of collagens IX and XI in this heteropolymer are not yet fully defined but, evidently, they are critically important since mutations in COLIX and COLXI genes result in chondrodysplasia phenotypes that feature precocious osteoarthritis. Collagens XII and XIV are thought also to be bound to fibril surfaces but not covalently attached. Collagen VI polymerizes into its own type of filamentous network that has multiple adhesion domains for cells and other matrix components. Collagen X is normally restricted to the thin layer of calcified cartilage that interfaces articular cartilage with bone.
Figures
References
-
- Lane JM, Weiss C. Review of articular cartilage collagen research. Arth Rheum. 1975;18:553–562. - PubMed
-
- Benninghoff A. Form und bau der Gelenknorpel in ihren Bezeihungen zur Funktion. Z Zellforsch Mikrosk Anat. 1925;2:783–825.
-
- Notzli H, Clark J. Deformation of loaded articular cartilage prepared for scanning electronmicroscopy with rapid freezing and freeze-substituion fixation. J Orthop Res. 1997;15:76–86. - PubMed
-
- Eyre DR, Brickley-Parsons DM, Glimcher MJ. Predominance of type I collagen at the surface of avian articular cartilage. FEBS Lett. 1978;85:259–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

