Conserved organization of centromeric chromatin in flies and humans
- PMID: 11879637
- PMCID: PMC3192492
- DOI: 10.1016/s1534-5807(02)00135-1
Conserved organization of centromeric chromatin in flies and humans
Abstract
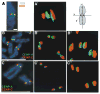
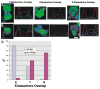
Recent studies have highlighted the importance of centromere-specific histone H3-like (CENP-A) proteins in centromere function. We show that Drosophila CID and human CENP-A appear at metaphase as a three-dimensional structure that lacks histone H3. However, blocks of CID/CENP-A and H3 nucleosomes are linearly interspersed on extended chromatin fibers, and CID is close to H3 nucleosomes in polynucleosomal preparations. When CID is depleted by RNAi, it is replaced by H3, demonstrating flexibility of centromeric chromatin organization. Finally, contrary to models proposing that H3 and CID/CENP-A nucleosomes are replicated at different times in S phase, we show that interspersed H3 and CID/CENP-A chromatin are replicated concurrently during S phase in humans and flies. We propose that the unique structural arrangement of CID/CENP-A and H3 nucleosomes presents centromeric chromatin to the poleward face of the condensing mitotic chromosome.
Figures




References
-
- Bernard P, Maure JF, Partridge JF, Genier S, Javerzat JP, Allshire RC. Requirement of heterochromatin for cohesion at centromeres. Science. 2001;294:2539–2542. - PubMed
-
- Blat Y, Kleckner N. Cohesins bind to preferential sites along yeast chromosome III, with differential regulation along arms versus the centric region. Cell. 1999;98:249–259. - PubMed
-
- Buchwitz BJ, Ahmad K, Moore LL, Roth MB, Henikoff S. A histone-H3-like protein in C. elegans. Nature. 1999;401:547–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

