Activation of type II alveolar epithelial cells during acute endotoxemia
- PMID: 11880315
- PMCID: PMC4015347
- DOI: 10.1152/ajplung.00217.2001
Activation of type II alveolar epithelial cells during acute endotoxemia
Abstract
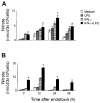
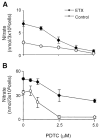
Lung injury induced by acute endotoxemia is associated with increased generation of inflammatory mediators such as nitric oxide and eicosanoids, which have been implicated in the pathophysiological process. Although production of these mediators by alveolar macrophages (AM) has been characterized, the response of type II cells is unknown and was assessed in the present studies. Acute endotoxemia caused a rapid (within 1 h) and prolonged (up to 48 h) induction of nitric oxide synthase-2 (NOS-2) in type II cells but a delayed response in AM (12-24 h). In both cell types, this was associated with increased nitric oxide production. Although type II cells, and to a lesser extent AM, constitutively expressed cyclooxygenase-2, acute endotoxemia did not alter this activity. Endotoxin administration had no effect on mitogen-activated protein kinase or protein kinase B-alpha (PKB-alpha) expression. However, increases in phosphoinositide 3-kinase and phospho-PKB-alpha were observed in type II cells. The finding that this was delayed for 12-24 h suggests that these proteins do not play a significant role in the regulation of NOS-2 in this model. After endotoxin administration to rats, a rapid (within 1-2 h) activation of nuclear factor-kappaB was observed. This response was transient in type II cells but was sustained in AM. Interferon regulatory factor-1 (IRF-1) was also activated rapidly in type II cells. In contrast, IRF-1 activation was delayed in AM. These data demonstrate that type II cells, like AM, are highly responsive during acute endotoxemia and may contribute to pulmonary inflammation.
Figures





Similar articles
-
Regulation of cyclooxygenase-2 by nitric oxide in activated hepatic macrophages during acute endotoxemia.J Leukoc Biol. 2002 Jun;71(6):1005-11. J Leukoc Biol. 2002. PMID: 12050186
-
Cyclooxygenase-2 downregulates inducible nitric oxide synthase in rat intestinal epithelial cells.Am J Physiol Gastrointest Liver Physiol. 2001 Sep;281(3):G688-96. doi: 10.1152/ajpgi.2001.281.3.G688. Am J Physiol Gastrointest Liver Physiol. 2001. PMID: 11518681
-
Induction of cyclooxygenase-2 by heat shock protein 60 in macrophages and endothelial cells.Am J Physiol Cell Physiol. 2002 Oct;283(4):C1267-77. doi: 10.1152/ajpcell.00609.2001. Am J Physiol Cell Physiol. 2002. PMID: 12225989
-
Alteration in heme oxygenase-1 and nitric oxide synthase-2 gene expression during endotoxemia in cyclooxygenase-2-deficient mice.Antioxid Redox Signal. 2004 Oct;6(5):850-7. doi: 10.1089/ars.2004.6.850. Antioxid Redox Signal. 2004. PMID: 15345145 Review.
-
Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation.Mutat Res. 2001 Sep 1;480-481:243-68. doi: 10.1016/s0027-5107(01)00183-x. Mutat Res. 2001. PMID: 11506818 Review.
Cited by
-
Protective Role of Surfactant Protein-D Against Lung Injury and Oxidative Stress Induced by Nitrogen Mustard.Toxicol Sci. 2018 Nov 1;166(1):108-122. doi: 10.1093/toxsci/kfy188. Toxicol Sci. 2018. PMID: 30060251 Free PMC article.
-
Exosomes Derived From Alveolar Epithelial Cells Promote Alveolar Macrophage Activation Mediated by miR-92a-3p in Sepsis-Induced Acute Lung Injury.Front Cell Infect Microbiol. 2021 May 10;11:646546. doi: 10.3389/fcimb.2021.646546. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34041043 Free PMC article.
-
Pneumonia-induced sepsis in mice: temporal study of inflammatory and cardiovascular parameters.Int J Exp Pathol. 2013 Apr;94(2):144-55. doi: 10.1111/iep.12016. Epub 2013 Feb 27. Int J Exp Pathol. 2013. PMID: 23441627 Free PMC article.
-
Pulmonary effects of inhaled limonene ozone reaction products in elderly rats.Toxicol Appl Pharmacol. 2007 Jul 15;222(2):211-20. doi: 10.1016/j.taap.2007.05.003. Epub 2007 May 21. Toxicol Appl Pharmacol. 2007. PMID: 17610924 Free PMC article.
-
Alveolar Epithelial Cell-Derived Mediators: Potential Direct Regulators of Large Airway and Vascular Responses.Am J Respir Cell Mol Biol. 2017 Jun;56(6):694-699. doi: 10.1165/rcmb.2016-0151PS. Am J Respir Cell Mol Biol. 2017. PMID: 28080134 Free PMC article. Review.
References
-
- Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974;30:35–42. - PubMed
-
- Amory-Rivier CF, Mohler J, Bedos JP, Azoulay-Dupuis E, Henin D, Muffat-Joly M, Carbon C, Moine P. Nuclear factor-kappaB activation in mouse lung lavage cells in response to Streptococcus pneumoniae pulmonary infection. Crit Care Med. 2000;28:3249–3256. - PubMed
-
- Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–1046. - PubMed
-
- Blackwell TS, Lancaster LH, Blackwell TR, Venkatakrishnan A, Christman JW. Differential NF-κB activation after intratracheal endotoxin. Am J Physiol Lung Cell Mol Physiol. 1999;277:L823–L830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

