Physiological role of calcium-activated potassium currents in the rat lateral amygdala
- PMID: 11880492
- PMCID: PMC6758860
- DOI: 10.1523/JNEUROSCI.22-05-01618.2002
Physiological role of calcium-activated potassium currents in the rat lateral amygdala
Abstract
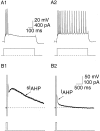
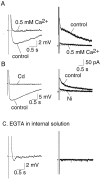
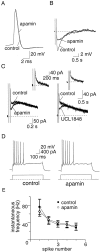
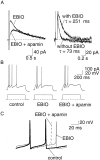
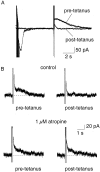
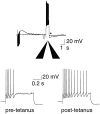
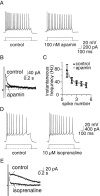
Principal neurons in the lateral nucleus of the amygdala (LA) exhibit a continuum of firing properties in response to prolonged current injections ranging from those that accommodate fully to those that fire repetitively. In most cells, trains of action potentials are followed by a slow afterhyperpolarization (AHP) lasting several seconds. Reducing calcium influx either by lowering concentrations of extracellular calcium or by applying nickel abolished the AHP, confirming it is mediated by calcium influx. Blockade of large conductance calcium-activated potassium channel (BK) channels with paxilline, iberiotoxin, or TEA revealed that BK channels are involved in action potential repolarization but only make a small contribution to the fast AHP that follows action potentials. The fast AHP was, however, markedly reduced by low concentrations of 4-aminopyridine and alpha-dendrotoxin, indicating the involvement of voltage-gated potassium channels in the fast AHP. The medium AHP was blocked by apamin and UCL1848, indicating it was mediated by small conductance calcium-activated potassium channel (SK) channels. Blockade of these channels had no effect on instantaneous firing. However, enhancement of the SK-mediated current by 1-ethyl-2-benzimidazolinone or paxilline increased the early interspike interval, showing that under physiological conditions activation of SK channels is insufficient to control firing frequency. The slow AHP, mediated by non-SK BK channels, was apamin-insensitive but was modulated by carbachol and noradrenaline. Tetanic stimulation of cholinergic afferents to the LA depressed the slow AHP and led to an increase in firing. These results show that BK, SK, and non-BK SK-mediated calcium-activated potassium currents are present in principal LA neurons and play distinct physiological roles.
Figures



References
-
- Benardo LS, Prince DA. Ionic mechanisms of cholinergic excitation in mammalian hippocampal pyramidal cells. Brain Res. 1982;249:333–344. - PubMed
-
- Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterisation of novel large conductance calcium-activated potassium channel β subunits, hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275:6453–6461. - PubMed
-
- Castle NA, Haylett DG, Jenkinson DH. Toxins in the characterization of potassium channels. Trends Neurosci. 1989;12:59–65. - PubMed
-
- Chen J-Q, Galanakis D, Ganellin CR, Dunn PM, Jenkinson DH. bis-Quinolinium cyclophanes: 8,14-diaza-1,7(1,4)-diquinolinacyclotetradecaphane (UCL 1848), a highly potent, selective, nonpeptidic blocker of the apamin-sensitive Ca2+-activated K+ channel. J Med Chem. 2000;43:3478–3481. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
