Evidence for a centrally located gate in the pore of a serotonin-gated ion channel
- PMID: 11880493
- PMCID: PMC6758895
- DOI: 10.1523/JNEUROSCI.22-05-01629.2002
Evidence for a centrally located gate in the pore of a serotonin-gated ion channel
Abstract
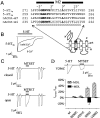
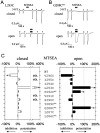
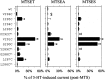
Serotonin-gated ion channels (5-HT3) are members of the ligand-gated channel family, which includes channels that are opened directly by the neurotransmitter acetylcholine, GABA, glycine, or glutamate. Although there is general agreement that the second transmembrane domain (M2) lines the pore, the position of the gate in the M2 is less certain. Here, we used substituted cysteine accessibility method (SCAM) to provide new evidence for a centrally located gate that moves during channel activation. In the closed state, three cysteine substitutions, located on the extracellular side of M2, were modified by methanethiosulfonate (MTS) reagents. In contrast, 13 cysteine substitutions were modified in the open state with MTS reagents. The pattern of inhibition (every three to four substitutions) was consistent with an alpha helical structure for the middle and cytoplasmic segments of the M2 transmembrane domain. Unexpectedly, open-state modification of two amino acids in the center of M2 with three different MTS reagents prevented channels from fully closing in the absence of neurotransmitter. Our results are consistent with a model in which the central region of the M2 transmembrane domain is inaccessible in the closed state and moves during channel activation.
Figures





Similar articles
-
Change of pore helix conformational state upon opening of cyclic nucleotide-gated channels.Neuron. 2000 Dec;28(3):899-909. doi: 10.1016/s0896-6273(00)00162-8. Neuron. 2000. PMID: 11163275
-
Structural determinants of the closed KCa3.1 channel pore in relation to channel gating: results from a substituted cysteine accessibility analysis.J Gen Physiol. 2007 Apr;129(4):299-315. doi: 10.1085/jgp.200609726. Epub 2007 Mar 12. J Gen Physiol. 2007. PMID: 17353352 Free PMC article.
-
Localization of the activation gate for small conductance Ca2+-activated K+ channels.J Neurosci. 2002 Aug 1;22(15):6499-506. doi: 10.1523/JNEUROSCI.22-15-06499.2002. J Neurosci. 2002. PMID: 12151529 Free PMC article.
-
The molecular basis of the structure and function of the 5-HT3 receptor: a model ligand-gated ion channel (review).Mol Membr Biol. 2002 Jan-Mar;19(1):11-26. doi: 10.1080/09687680110110048. Mol Membr Biol. 2002. PMID: 11989819 Review.
-
Pore structure of the Cys-loop ligand-gated ion channels.Neurochem Res. 2009 Oct;34(10):1805-15. doi: 10.1007/s11064-009-9971-2. Epub 2009 Apr 19. Neurochem Res. 2009. PMID: 19381804 Review.
Cited by
-
A single channel mutation alters agonist efficacy at 5-HT3A and 5-HT3AB receptors.Br J Pharmacol. 2013 Sep;170(2):391-402. doi: 10.1111/bph.12287. Br J Pharmacol. 2013. PMID: 23822584 Free PMC article.
-
One-microsecond molecular dynamics simulation of channel gating in a nicotinic receptor homologue.Proc Natl Acad Sci U S A. 2010 Apr 6;107(14):6275-80. doi: 10.1073/pnas.1001832107. Epub 2010 Mar 22. Proc Natl Acad Sci U S A. 2010. PMID: 20308576 Free PMC article.
-
Pathways and Barriers for Ion Translocation through the 5-HT3A Receptor Channel.PLoS One. 2015 Oct 14;10(10):e0140258. doi: 10.1371/journal.pone.0140258. eCollection 2015. PLoS One. 2015. PMID: 26465896 Free PMC article.
-
Structure, Function and Physiology of 5-Hydroxytryptamine Receptors Subtype 3.Subcell Biochem. 2021;96:373-408. doi: 10.1007/978-3-030-58971-4_11. Subcell Biochem. 2021. PMID: 33252737 Review.
-
The L293 residue in transmembrane domain 2 of the 5-HT3A receptor is a molecular determinant of allosteric modulation by 5-hydroxyindole.Neuropharmacology. 2008 Jun;54(8):1153-65. doi: 10.1016/j.neuropharm.2008.03.009. Epub 2008 Mar 27. Neuropharmacology. 2008. PMID: 18436267 Free PMC article.
References
-
- Akabas MH, Kaufmann C, Archdeacon P, Karlin A. Identification of acetylcholine receptor channel-lining residues in the entire M2 segment of the α subunit. Neuron. 1994;13:919–927. - PubMed
-
- Ballesteros JA, Shi L, Javitch JA. Structural mimicry in G protein-coupled receptors: implications of the high-resolution structure of rhodopsin for structure-function analysis of rhodopsin-like receptors. Mol Pharmacol. 2001;60:1–19. - PubMed
-
- Bertrand S, Devillers-Thiery A, Palma E, Buisson B, Edelstein SJ, Corringer P-J, Changeux J-P, Bertrand D. Paradoxical allosteric effects of competitive inhibitors on neuronal α7 nicotinic receptor mutants. Mol Neurosci. 1997;8:3591–3596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
