The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease
- PMID: 11880515
- PMCID: PMC6758862
- DOI: 10.1523/JNEUROSCI.22-05-01858.2002
The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease
Abstract
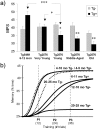
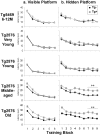
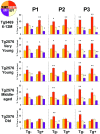
Transgenic mice expressing mutant amyloid precursor proteins (APPs) have provided important new information about the pathogenesis of Alzheimer's disease (AD) histopathology. However, the molecular basis of memory loss in these mice is poorly understood. One of the major impediments has been the difficulty of distinguishing between age-dependent and age-independent behavioral changes. To address this issue we studied in parallel two lines of APP transgenic mice expressing comparable levels of mutant and wild-type human APP. This enabled us to identify age-independent behavioral deficits that were not specifically related to mutant APP expression. When mice with age-independent deficits were eliminated, we detected memory loss in transgenic mice expressing mutant APP (Tg2576 mice) starting at approximately 6 months, which coincided with the appearance of detergent-insoluble Abeta aggregates (Abeta(insol)). Genetically accelerating the formation of Abeta(insol) resulted in an earlier onset of memory decline. A facile interpretation of these results, namely that memory loss and Abeta(insol) were closely connected, was rejected when we extended our analysis to include older mice. No obvious correspondence between memory and Abeta(insol) was apparent in a combined group of old and young mice unless the mice were stratified by age, whereupon inverse correlations between memory and Abeta(insol) became evident. These results suggested that Abeta(insol) is a surrogate marker for small assemblies of Abeta that disrupt cognition and occur as intermediates during Abeta(insol) formation, and they are the first descriptive in vivo data supporting their role in impairing memory. These studies also provide a methodological framework within which to investigate these Abeta assemblies in vivo.
Figures




References
-
- Arriagada PV, Growdon JH, Hedley-White ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. - PubMed
-
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA. 1999;96:5280–5285. - PMC - PubMed
-
- Bartoo GT, Nochlin D, Chang D, Kim Y, Sumi SM. The mean Aβ load in the hippocampus correlates with duration and severity of dementia in subgroups of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:531–540. - PubMed
-
- Bellush LL, Wright AM, Walker JP, Kopchick J, Colvin RA. Caloric restriction and spatial learning in old mice. Physiol Behav. 1996;60:541–547. - PubMed
-
- Berg L, McKeel DW, Jr, Miller JP, Baty J, Morris JC. Neuropathological indexes of Alzheimer's disease in demented and nondemented persons aged 80 years and older. Arch Neurol. 1993;50:349–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
