Neuronal activity in substantia nigra pars reticulata during target selection
- PMID: 11880518
- PMCID: PMC6758865
- DOI: 10.1523/JNEUROSCI.22-05-01883.2002
Neuronal activity in substantia nigra pars reticulata during target selection
Abstract
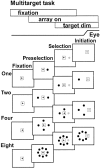
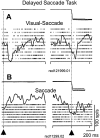
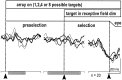
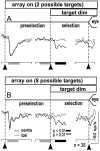
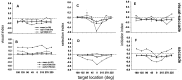
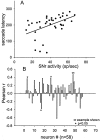
Complex visual scenes require that a target for an impending saccadic eye movement be selected from a number of possible targets. We investigated whether changing the number of stimuli from which a target would be identified altered the activity of substantia nigra pars reticulata (SNr) neurons of the basal ganglia (BG) and how such changes might contribute to changes we observed previously in the superior colliculus (SC). One, two, four, or eight visual stimuli appeared on random trials while monkeys fixated a centrally located spot. After a delay, one of the stimuli in the array changed luminance, indicating that it was the saccade target. We found that SNr neurons that had a pause in tonic activity after target onset and when the saccade was made to the target showed a modulation of activity during the multitarget task. Because the number of stimuli in the array increased from one to eight, the initial pause after the onset of the visual stimulus decreased. Activity during the preselection delay was reduced but was independent of the number of possible targets present. When one of the stimuli was identified as the saccade target, but before the saccade was made, we found a sharp decline in activity. This decline was related to the monkey's selecting the target rather than the luminance change identifying the target, because on error trials, when the luminance changed but a saccade was not made to the target, the activity did not decline. The decline for the preferred target location was also accompanied by a lesser decline for adjacent locations. Our findings indicate that SNr activity changes with target selection as it does with saccade initiation and that the SNr could make substantial, direct contributions to the SC at both times. The pause in SNr activity with target selection is consistent with the hypothesis that BG provide a disinhibition for the selection of desired movements.
Figures




References
-
- Anderson ME, Yoshida M. Electrophysiological evidence for branching nigral projections to the thalamus and the superior colliculus. Brain Res. 1977;137:361–375. - PubMed
-
- Anderson ME, Yoshida M. Axonal branching patterns and location of nigrothalamic and nigrocollicular neurons in the cat. J Neurophysiol. 1980;43:883–895. - PubMed
-
- Basso MA, Wurtz RH. Modulation of neuronal activity by target uncertainty. Nature. 1997;389:66–69. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
