Persistent, exocytosis-independent silencing of release sites underlies homosynaptic depression at sensory synapses in Aplysia
- PMID: 11880525
- PMCID: PMC6758855
- DOI: 10.1523/JNEUROSCI.22-05-01942.2002
Persistent, exocytosis-independent silencing of release sites underlies homosynaptic depression at sensory synapses in Aplysia
Erratum in
- J Neurosci 2002 Apr 15;22(8):3302
Abstract
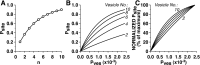
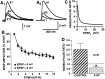
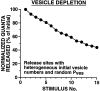
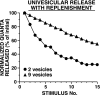
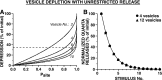
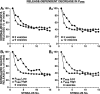
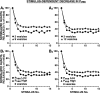
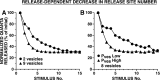
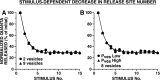
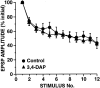
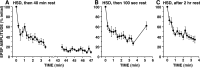
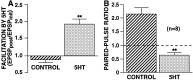
The synaptic connections of Aplysia sensory neurons (SNs) undergo dramatic homosynaptic depression (HSD) with only a few low-frequency stimuli. Strong and weak SN synapses, although differing in their probabilities of release, undergo HSD at the same rate; this suggests that the major mechanism underlying HSD in these SNs may not be depletion of the releasable pool of vesicles. In computational models, we evaluated alternative mechanisms of HSD, including vesicle depletion, to determine which mechanisms enable strong and weak synapses to depress with identical time courses. Of five mechanisms tested, only release-independent, stimulus-dependent switching off of release sites resulted in HSD that was independent of initial synaptic strength. This conclusion that HSD is a release-independent phenomenon was supported by empirical results: an increase in Ca2+ influx caused by spike broadening with a K+ channel blocker did not alter HSD. Once induced, HSD persisted during 40 min of rest with no detectable recovery; thus, release does not recover automatically with rest, contrary to what would be expected if HSD represented an exhaustion of the exocytosis mechanism. The hypothesis that short-term HSD involves primarily a stepwise silencing of release sites, rather than vesicle depletion, is consistent with our earlier observation that HSD is accompanied by only a modest decrease in release probability, as indicated by little change in the paired-pulse ratio. In contrast, we found that there was a dramatic decrease in the paired-pulse ratio during serotonin-induced facilitation; this suggests that heterosynaptic facilitation primarily involves an increase in release probability, rather than a change in the number of functional release sites.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
