Highly dissimilar behaviors mediated by a multifunctional network in the marine mollusk Tritonia diomedea
- PMID: 11880529
- PMCID: PMC6758888
- DOI: 10.1523/JNEUROSCI.22-05-01985.2002
Highly dissimilar behaviors mediated by a multifunctional network in the marine mollusk Tritonia diomedea
Abstract
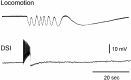
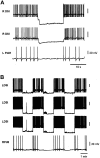
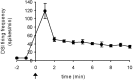
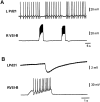
Several motor networks have now been found to be multifunctional, in which one group of neurons participates in the generation of multiple behavioral motor programs. Not surprisingly, the behaviors involved are frequently closely related, often using the same or similar muscle groups. Here we describe an interneuronal network in the marine mollusk Tritonia diomedea that is involved in producing two highly dissimilar behaviors, rhythmic, muscle-based escape swimming and nonrhythmic, cilia-mediated crawling. Several observations support this conclusion. First, the dorsal swim interneurons (DSIs) of the swim central pattern generator (CPG) directly excite Pedal neuron 21 (Pd21) and Pd5, the only identified cilia-activating efferent neurons in Tritonia. Second, stimulation of a single DSI elicits beating of the foot cilia in semi-intact preparations and crawling in intact animal treadmill preparations. Third, the DSIs fire at an elevated rate for nearly 1 hr after a swim motor program, which correlates reasonably well with the period freely behaving animals were found to crawl after they swam. Fourth, silencing the tonically active DSIs after a swim motor program substantially reduces or eliminates ongoing cilia neuron firing, indicating that the DSIs are major contributors to the synaptic input driving these cells. Finally, all of the other swim CPG neurons also connect to the cilia neurons, most monosynaptically. Taken together, these observations indicate that the Tritonia swim CPG network participates in producing both escape swimming and crawling. Given the extreme differences between these behaviors---rhythmic versus tonic, muscular versus ciliary, and brief versus prolonged--these findings reveal a striking versatility for a small multifunctional network.
Figures




References
-
- Audesirk G. Central neuronal control of cilia in Tritonia diomedea. Nature. 1978a;272:541–543. - PubMed
-
- Audesirk G. Properties of central motor neurons exciting locomotory cilia in Tritonia diomedea. J Comp Physiol [A] 1978b;128:259–267.
-
- Audesirk G, Audesirk T. Complex mechanoreceptors in Tritonia diomedea. II. Neuronal correlates of a change in behavioral responsiveness. J Comp Physiol [A] 1980;141:111–122.
-
- Audesirk G, McCaman RE, Willows AOD. The role of serotonin in the control of pedal ciliary activity by identified neurons in Tritonia diomedea. Comp Biochem Physiol C. 1979;62C:87–91. - PubMed
-
- Brown G, Frost WN, Getting PA. Habituation and iterative enhancement of multiple components of the Tritonia swim response. Behav Neurosci. 1996;110:478–485. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
