Local generation of fast ripples in epileptic brain
- PMID: 11880532
- PMCID: PMC6758883
- DOI: 10.1523/JNEUROSCI.22-05-02012.2002
Local generation of fast ripples in epileptic brain
Abstract
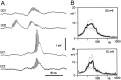
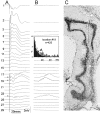
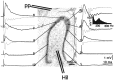
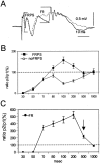
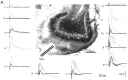
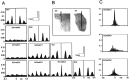
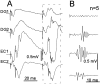
Aperiodic high-frequency oscillations (>100 Hz) reflect a short-term synchronization of neuronal electrical activity. It has been shown in the epileptic brain that spontaneous oscillations in the frequency range of 250-600 Hz reflect action potential population bursts of synchronously discharging neuronal clusters. These oscillations occur in the early stages of epileptogenesis in areas adjacent to the brain lesion and may trigger the formation of seizure-generating neuronal networks. We studied the extent of the area generating oscillations in the frequency range of 250-600 Hz [fast ripples (FRs)] in intrahippocampal kainic acid-treated rats with spontaneous seizures, by analyzing voltage versus depth profiles of FRs in hippocampal and parahippocampal areas in freely moving animals and by spatial mapping in hippocampal slice preparations in vitro. The strength of inhibition was compared in areas with and without FRs using a paired-pulse paradigm. The extent of the areas generating FRs did not exceed 1 mm(3). The areas generating FRs became broader after the application of the GABA(A) receptor antagonist bicuculline. Paired-pulse fast inhibition at 15-30 msec intervals was similar in areas generating FRs and areas not generating FRs. Our data illustrate that hypothesized clusters of highly interconnected neurons are capable of overcoming interneuron feedback inhibition, resulting in generation of epileptiform bursts, eventually leading to seizure activity.
Figures



References
-
- Bertram EH, Zhang DX, Mangan P, Fountain N, Rempe D. Functional anatomy of limbic epilepsy: a proposal for central synchronization of a diffusely hyperexcitable network. Epilepsy Res. 1998;32:194–205. - PubMed
-
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999a;40:127–137. - PubMed
-
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999b;9:137–142. - PubMed
-
- Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41:S144–S152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
