13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS)
- PMID: 11880625
- PMCID: PMC122412
- DOI: 10.1073/pnas.052005699
13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS)
Abstract
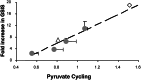
Cellular metabolism of glucose is required for stimulation of insulin secretion from pancreatic beta cells, but the precise metabolic coupling factors involved in this process are not known. In an effort to better understand mechanisms of fuel-mediated insulin secretion, we have adapted 13C NMR and isotopomer methods to measure influx of metabolic fuels into the tricarboxylic acid (TCA) cycle in insulinoma cells. Mitochondrial metabolism of [U-13C3]pyruvate, derived from [U-13C6]glucose, was compared in four clonal rat insulinoma cell 1-derived cell lines with varying degrees of glucose responsiveness. A 13C isotopomer analysis of glutamate isolated from these cells showed that the fraction of acetyl-CoA derived from [U-13C6]glucose was the same in all four cell lines (44 +/- 5%, 70 +/- 3%, and 84 +/- 4% with 3, 6, or 12 mM glucose, respectively). The 13C NMR spectra also demonstrated the existence of two compartmental pools of pyruvate, one that exchanges with TCA cycle intermediates and a second pool derived from [U-13C6]glucose that feeds acetyl-CoA into the TCA cycle. The 13C NMR spectra were consistent with a metabolic model where the two pyruvate pools do not randomly mix. Flux between the mitochondrial intermediates and the first pool of pyruvate (pyruvate cycling) varied in proportion to glucose responsiveness in the four cell lines. Furthermore, stimulation of pyruvate cycling with dimethylmalate or its inhibition with phenylacetic acid led to proportional changes in insulin secretion. These findings indicate that exchange of pyruvate with TCA cycle intermediates, rather than oxidation of pyruvate via acetyl-CoA, correlates with glucose-stimulated insulin secretion.
Figures




References
-
- Newgard C B, McGarry J D. Annu Rev Biochem. 1995;64:689–719. - PubMed
-
- Newgard C B, Matschinsky F M. In: Handbook of Physiology. Jefferson J, Cherrington A, editors. Vol. 2. London: Oxford Univ. Press; 2001. pp. 125–152.
-
- MacDonald M J. Arch Biochem Biophys. 1995;319:128–132. - PubMed
-
- MacDonald M J, McKenzie D I, Walker T M, Kaysen J H. Horm Metab Res. 1992;24:158–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

