Different contributions of thymopoiesis and homeostasis-driven proliferation to the reconstitution of naive and memory T cell compartments
- PMID: 11880642
- PMCID: PMC122460
- DOI: 10.1073/pnas.052714099
Different contributions of thymopoiesis and homeostasis-driven proliferation to the reconstitution of naive and memory T cell compartments
Abstract
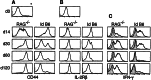
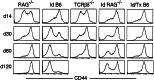
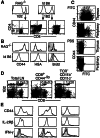
Following transfer into lymphopenic hosts, naive CD8 T cells proliferate and acquire memory phenotype. Although the acquired phenotype is stable in recombination activating gene-1-deficient (RAG-/-) recipients, in sublethally irradiated mice naive CD8 T cells of donor origin gradually accumulate. The naive cells have been attributed to phenotypic reversion of homeostatic memory cells, implying instability of memory phenotype and restoration of the naive T cell compartment by homeostasis-driven proliferation. We show here that (i) the accumulation of naive CD8 T cells of donor origin only occurs in recipients that have been irradiated and have an intact thymus; (ii) the apparent reversion of memory to naive cells actually results from de novo T cell development of hematopoietic stem cells, present in the donor spleen or lymph node cell populations, in the thymus of irradiated recipients; and (iii) the number of homeostatic memory cells generated in both RAG-/- and irradiated hosts reaches a plateau value and their phenotype is stably maintained even after retransfer into nonirradiated normal mice for 30 days. These findings demonstrate that homeostatic memory T cells do not revert to naive cells. After severe T cell depletion homeostasis-driven proliferation restores only the memory T cell compartment, whereas thymopoiesis is required for the reconstitution of the naive T cell compartment.
Figures


References
-
- Goldrath A W, Bevan M J. Nature (London) 1999;402:255–261. - PubMed
-
- Marrack P, Bender J, Hildeman D, Jordan M, Mitchell T, Murakami M, Sakamoto A, Schaefer B C, Swanson B, Kappler J. Nat Immunol. 2000;1:107–111. - PubMed
-
- Mackall C L, Hakim F T, Gress R E. Semin Immunol. 1997;9:339–346. - PubMed
-
- Tanchot C, Lemonnier F A, Pérarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

