A stilbene synthase from Japanese red pine (Pinus densiflora): implications for phytoalexin accumulation and down-regulation of flavonoid biosynthesis
- PMID: 11880657
- PMCID: PMC122519
- DOI: 10.1073/pnas.042698899
A stilbene synthase from Japanese red pine (Pinus densiflora): implications for phytoalexin accumulation and down-regulation of flavonoid biosynthesis
Abstract
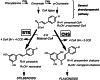
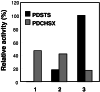
Stilbene synthase (STS) and chalcone synthase (CHS) are plant-specific polyketide synthases that play key roles in the stilbenoid and flavonoid biosyntheses, respectively. We have recently isolated from Pinus densiflora three STS cDNAs (PDSTS1, PDSTS2, and PDSTS3) and one CHS cDNA (PDCHSX). We then heterologously expressed these cDNAs in Escherichia coli and characterized their properties. An unusual STS isozyme, PDSTS3, lacks the common C-terminal extension of STS because of a frame-shift mutation and shows the highest pinosylvin-forming activity among the STSs tested. Pinosylvin was shown to be a potent inhibitor of PDCHSX (K(i) = 6 microM) as well as PDSTS2 (K(i) = 13 microM), which presumably maintains the balance between the stilbenoid and flavonoid biosyntheses. PDSTS3 was insensitive to product inhibition. We identified PDSTS3 in the pine seedlings as well as full-length STS. The data provide evidence that PDSTS3 is involved in the potential regulation of the stilbenoid and flavonoid biosynthetic pathways in pine trees.
Figures


References
-
- Mamiya Y. In: Pathogenicity of the Pine Wood Nematode. Wingfield M J, editor. St. Paul: Am. Phytopathol. Soc.; 1987. pp. 59–65.
-
- Suga T, Ohta S, Munesada K, Ide N, Kurokawa M, Shimizu M, Ohta E. Phytochemistry. 1993;33:1395–1401.
-
- Tröpf S, Lanz T, Rensing S A, Schröder J, Schröder G. J Mol Evol. 1994;38:610–618. - PubMed
-
- Kao C M, Katz L, Khosla C. Science. 1994;265:509–512. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

